Effect of Santolina pectinata (Lag.) Essential Oil to protect against the corrosion of Mild steel in 1.0 M HCl: Experimental and quantum chemical studies
Abstract
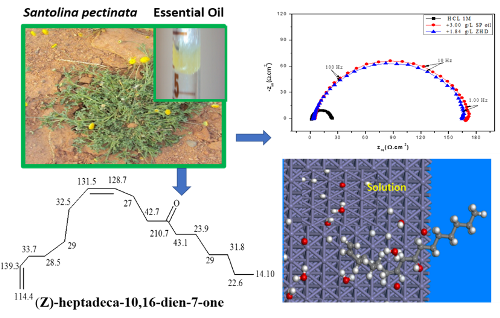
The essential oil of Santolina pectinata aerial parts (SP oil) was extracted by hydrodistillation and analyzed by Gas Chromatography (GC) and Gas Chromatography-Mass Spectrometry (GC/MS). 34 components were identified, accounting 59.4% of the total oil, which (Z)-heptadeca-10,16-dien-7-one (ZHD) was the major component with 28 % of the oil. The protective effect of this oil on the corrosion of mild steel (MS) in 1M HCl solution was tested by the measurements of Weight loss (WL), potentiodynamic polarization (PDP), electrochemical impedance spectroscopy (EIS). From WL measurements, the inhibiting effect was raised with the increasing of both concentration inhibitor and temperature. PDP curves led to a mixed-type inhibitor. The charge transfer process mainly controls the results of EIS measurements. The SP oil adsorption not only was discovered to obey Langmuir isotherm but showed a chemical interaction as well. The computational methods such as density functional theory (DFT), Monte Carlo (MC) and radial distribution function (RDF) simulations were also performed to analyze the possible contribution of (Z)-heptadeca-10,16-dien-7-one (ZHD) to the corrosion prevention effect of global oil.
Full Text:
PDFReferences
- M. G. Hosseini, M. Ehteshamzadeh, T. Shahrabi, Protection of mild steel corrosion with Schiff bases in 0.5 M H2SO4 solution, Electrochimica Acta, 2007, 52, 3680-3685.
- E. E. Oguzie, Y. Li, F. H. Wang, Effect of surface nanocrystallization on corrosion and corrosion inhibition of low carbon steel: Synergistic effect of methionine and iodide ion, Electrochimica Acta, 2007, 52, 6988-6996.
- G. Blustein, J. Rodriguez, R. Romanogli, C. F. Zinola, Inhibition of steel corrosion by calcium benzoate adsorption in nitrate solutions, Corrosion Science, 2005, 47, 369-383.
- M. Mihit, K. Laarej, H. A. El Makarim, L. Bazzi, R. Salghi, B. Hammouti, Study of the inhibition of the corrosion of copper and zinc in HNO3 solution by electrochemical technique and quantum chemical calculations, Arabian Journal of Chemistry, 2010, 3, 55-60.
- D. M. Jamil, A. K. Al-Okbi, S. B. Al-Baghdadi, A. A. Al-Amiery, A. Kadhim, T. S. Gaaz, A. A. H. Kadhum, A. B. Mohamad, Experimental and theoretical studies of Schiff bases as corrosion inhibitors, Chemistry Central Journal, 2018, 12, 1-9.
- A. Rubaye, A. Abdulwahid, S. B. Al-Baghdadi, A. Al-Amiery, A. Kadhum, A. Mohamad, Cheery sticks plant extract as a green corrosion inhibitor complemented with LC-EIS/MS spectroscopy, International Journal of Electrochemical Science, 2015, 10, 8200-8209.
- S. Junaedi, A. A. H. Kadhum, A. A. Al-Amiery, A. B. Mohamad, M. S. Takriff, Synthesis and characterization of novel corrosion inhibitor derived from oleic acid: 2-Amino 5-Oleyl-1, 3,
-Thiadiazol (AOT), International Journal of Electrochemical Science, 2012, 7, 3543-3554.
- A. Habibiyan, B. Ramezanzadeh, M. Mahdavian, M. Kasaeian, Facile size and chemistry-controlled synthesis of mussel-inspired bio-polymers based on Polydopamine Nanospheres: Application as eco-friendly corrosion inhibitors for mild steel against aqueous acidic solution, Journal of Molecular Liquids, 2020, 298, 111974.
- A. Dehghani, G. Bahlakeh, B. Ramezanzadeh, M. Ramezanzadeh, Potential role of a novel green eco-friendly inhibitor in corrosion inhibition of mild steel in HCl solution: Detailed macro/micro-scale experimental and computational explorations, Construction and Building Materials, 2020, 245, 118464.
- A. Dehghani, G. Bahlakeh, B. Ramezanzadeh, Green Eucalyptus leaf extract: a potent source of bio-active corrosion inhibitors for mild steel, Bioelectrochemistry, 2019, 130, 107339.
- Z. Sanaei, M. Ramezanzadeh, G. Bahlakeh, B. Ramezanzadeh, Use of Rosa canina fruit extract as a green corrosion inhibitor for mild steel in 1 M HCl solution: A complementary experimental, molecular dynamics and quantum mechanics investigation, Journal of industrial and engineering chemistry, 2019, 69, 18-31.
- A. Dehghani, G. Bahlakeh, B. Ramezanzadeh, M. Ramezanzadeh, Detailed macro-/micro-scale exploration of the excellent active corrosion inhibition of a novel environmentally friendly green inhibitor for carbon steel in acidic environments, Journal of the Taiwan Institute of Chemical Engineers, 2019, 100, 239-261.
- N. Asadi, M. Ramezanzadeh, G. Bahlakeh, B. Ramezanzadeh, Utilizing Lemon Balm extract as an effective green corrosion inhibitor for mild steel in 1M HCl solution: A detailed experimental, molecular dynamics, Monte Carlo and quantum mechanics study, Journal of the Taiwan Institute of Chemical Engineers, 2019, 95, 252-272.
- M. Znini, J. Paolini, L. Majidi, J.-M. Desjobert, J. Costa, N. Lahhit, A. Bouyanzer, Evaluation of the inhibitive effect of essential oil of Lavandula multifida L., on the corrosion behavior of C38 steel in 0.5 MH2SO4 medium, Research on Chemical Intermediates, 2012, 38, 669-683.
- M. Manssouri, Y. El Ouadi, M. Znini, J. Costa, A. Bouyanzer, J. M. Desjobert, L. Majidi, Adsorption proprieties and inhibition of mild steel corrosion in HCl solution by the essential oil from fruit of Moroccan Ammodaucus leucotrichus, Journal of Materials and Environmental Science, 2015, 6, 631-646.
- A. Ansari, M. Znini, A. Laghchimi, J. Costa, P. Ponthiaux, L. Majidi, Chemical composition, adsorption proprieties and corrosion inhibition on mild steel of Mentha rotundifolia L. essential oil from Morocco, Der Pharmacia Lettre, 2015, 7, 125-140.
- M. Manssouri, M. Znini, A. Ansari, A. Bouyenzer, Z. Faska, L. Majidi, Odorized and deodorized aqueous extracts of Ammodaucus leucotrichus fruits as green inhibitor for C38 steel in hydrochloric acid solution, Der Pharma Chemica, 2014, 6, 331-345.
- M. Znini, A. Ansari, J. Costa, O. Senhaji, J. Paolini, L. Majidi, Experimental, Quantum Chemical and Molecular Dynamic Simulations Studies on the Corrosion Inhibition of C38 Steel in 1M HCl by Anethum graveolens Essential Oil, Analytical and Bioanalytical Electrochemistry, 2019, 11, 1426-1451.
- A. Ansari, M. Manssouri, A. Laghchimi, M. Znini, Z. Lakbaibi, M. Azrour, Experimental and theoretical study on corrosion inhibition of new synthesized menthone derivatives (Menthopyrazole compounds) for mild steel in 1M HCl solution, Mediterranean Journal of Chemistry, 2020, 10, 62-76.
- S. López Udías, C. Fabregat, G. Mateo, Santolina ageratifolia" Barnades ex Asso (Compositae) y el agregado S. rosmarinifolia L." Santolina ageratifolia" Barnades ex Asso (Compositae) and their relationships with S. rosmarinifolia L, in: Anales Del Jardín Botánico de Madrid, Real Jardín Botánico, 1997, 55, 285-296.
- M. Lamrani Alaoui, F. García Novo, Etude comparative de la biodiversite des matorrals des Parcs Naturels de Grazalema (Espagne) et de la Talassemtane (Maroc), Annales de La Recherche Forestiere Au Maroc, 1999, 21-43.
- M. J. Pérez-Alonso, A. V. Negueruela, The essential oils of four Santolina species, Flavour and Fragrance Journal, 1988, 3, 37-42.
- R. Tundis, M. R. Loizzo, A Review of the Traditional Uses, Phytochemistry and Biological Activities of the Genus Santolina, Planta Medica, 2008, 84, 627-637.
-A. F. Barrero, R. Alvarez-Manzaneda, J. F. Quilez, M. M. Herrador, Sesquiterpenes from Santolina chamaecyparissus subsp. squarrosa, Phytochemistry, 1998, 48, 807-813.
- A. F. Barrero, M. M. Herrador, J. F. Quilez, R. Alvarez-Manzaneda, D. Portal, J. A. Gavin, D. G. Gravalos, M. S. J. Simmonds, W. M. Blaney, Bioactive sesquiterpenes from Santolina rosmarinifolia subsp. Canescens.
A conformational analysis of the germacrane ring, Phytochemistry, 1999, 51, 529-541.
- A. F. Barrero, M. M. Herrador, R. J. Álvarez-Manzaneda, M. Quirós, A. Lara, J. Quílez del Moral, Longipinene derivatives from Santolina viscosa, Journal of Natural Products, 2000, 63, 587-591.
- J. A. Marco, J. F. Sanz-cervera, M. Carda, J. Lex, Oxygenated germacranes from Santolina chamaecyparissus, Phytochemistry, 1993, 34,1549-1559.
- M. Fennane, M. I. Tattou, B. Valdés, Catalogue des plantes vasculaires rares, menacées ou endémiques du Maroc, Herbarium Mediterraneum Panormitanum, 1998.
- A. F. Barrero, J. F. Sánchez, E. Arana, Germacranolides from Santolina rosmarinifolia subsp. Canescens. Phytochemistry, 1988, 27, 3969-3970.
- M. Manssouri, A. Ansari, M. Znini, L. Majidi,
J. Costa, Chemical Composition of the Santolina pectinata Lag., essential oil from Morocco: Identification of (Z)-heptadeca-10, 16-dien-7-one as a new natural component, Egyptian Journal of Chemistry, 2020, 63, 51-58.
- R. G. Parr, W. Yang, International series of monographs on chemistry 16: Density-functional theory of atoms and molecules, Oxford University Press, New York, 1989, 142-168.
- M. J. Frisch et al., GAUSSIAN 09, Revision E. 01, Gaussian Inc., Wallingford CT, 2009.
- W. J. Hehre, R. Ditchfield, J. A. Pople, Self-consistent molecular orbital methods. XII. Further extensions of Gaussian-type basis sets for use in molecular orbital studies of organic molecules, Journal of Chemical Physics, 1972, 56, 2257-2261.
- P. C. Hariharan, J. A. Pople, The influence of polarization functions on molecular orbital hydrogenation energies, Theoretical Chemistry Accounts, 1973, 28, 213-222.
- L. R. Domingo, P. Pérez, J. A. Sáez, Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions, Royal Society of Chemistry Advances, 2013, 3,1486-1494.
- O. Dagdag, R. Hsissou, A. Berisha, H. Erramli, O. Hamed, S. Jodeh, A. El Harfi, Polymeric-Based Epoxy Cured with a Polyaminoamide as an Anticorrosive Coating for Aluminum 2024-T3 Surface: Experimental Studies Supported by Computational Modeling, Journal of Bio- and Tribo-Corrosion, 2019, 5, 58.
- L. Afia, O. Hamed, M. Larouj, H. Lgaz, S. Jodeh, R. Salghi, Novel natural-based diazepines as effective corrosion inhibitors for carbon steel in HCl solution: experimental, theoretical and Monte Carlo simulations, Transactions- Indian Institute of Metals, 2017, 70, 2319-2333.
- Materials Studio version 8.0; Accelrys Software Inc, San Diego, 2016.
- H. Sun, COMPASS: an ab initio force-field optimized for condensed-phase applications overview with details on alkane and benzene compounds, Journal of Physical Chemistry B, 1998, 102, 7338-7364.
- D. Frenkel, B. Smit, Understanding Molecular Simulation-From Algorithms to Applications, Computational sciences series, Elsevier (Academic Press), 2002, 1, 1-638.
- Z. Zhang, N. C. Tian, X. D. Huang, W. Shang, L. Wu, Synergistic inhibition of carbon steel corrosion in 0.5 M HCl solution by indigo carmine and some cationic organic compounds: experimental and theoretical studies, Royal Society of Chemistry Advances, 2016, 6,
-22268.
- S.-W. Xie, Z. Liu, G.-C. Han, W. Li, J. Liu, Z. Chen, Molecular dynamics simulation of inhibition mechanism of 3, 5-dibromo salicylaldehyde Schiff’s base, Computational and Theoretical Chemistry, 2015, 1063, 50-62.
- L. Kadiri, M. Galai, M. Ouakki, Y. Essaadaoui, A. Ouass, M. Cherkaoui, E.-H. Rifi, A. Lebkiri, Coriandrum Sativum. L Seeds Extract as a Novel Green Corrosion Inhibitor for Mild Steel in 1.0 M Hydrochloric and 0.5 M Sulfuric Solutions, Analytical and Bioanalytical Electrochemistry, 2018, 10, 249-268.
- A. K. Satapathy, G. Gunasekaran, S. C. Sahoo, K. Amit, P. V. Rodrigues, Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution, Corrosion Science, 2009, 51, 2848-2856.
- A.R. Sathiya Priya, V.S. Muralidharan, A. Subramania, Development of novel acidizing inhibitors for carbon steel corrosion in 15% boiling hydrochloric acid, Corrosion, 2008, 64, 541-552.
- L. Larabi, Y. Harek, M. Traisnel, A. Mansri, Synergistic influence of poly (4-vinylpyridine) and potassium iodide on inhibition of corrosion of mild steel in 1M HCl, Journal of Applied Electrochemistry, 2004, 34, 833-839.
- F. Mansfeld, M.W. Kendig, S. Tsai, Synergistic influence of poly (4-vinylpyridine) and potassium iodide on inhibition of corrosion of mild steel in 1 M HCl, Corrosion, 1982, 38, 570.
- K. Jüttner, Electrochemical impedance spectroscopy (EIS) of corrosion processes on inhomogeneous surfaces, Electrochimica Acta, 1990, 35, 1501-1508.
- M. Benabdellah, A. Aouniti, A. Dafali, B. Hammouti, M. Benkaddour, A. Yahyi, A. Ettouhami, Investigation of the inhibitive effect of triphenyltin 2-thiophene carboxylate on corrosion of steel in 2 M H3PO4 solutions, Applied Surface Science, 2006, 252, 8341-8347.
- L. Messaadia, O. I. D. El Mouden, A. Anejjar, M. Messali, R. Salghi, O. Benali, O. Cherkaoui, A. Lallam, Adsorption and corrosion inhibition of new synthesized Pyridazinium-Based Ionic Liquid on Carbon steel in 0.5 M H2SO4, Journal of Materials and Environmental Science, 2015, 6, 598-606.
- A. M. Badiea, K. N. Mohana, Effect of temperature and fluid velocity on corrosion mechanism of low carbon steel in presence of
-hydrazino-4, 7-dimethylbenzothiazole in industrial water medium, Corrosion Science, 2015, 51, 2231-2241.
- E. Khamis, F. Bellucci, R. M. Latanision, E. S. H. El-Ashry, Acid corrosion inhibition of nickel by 2-(triphenosphoranylidene) succinic anhydride, Corrosion, 1991,47, 677-686.
- F. Bentiss, M. Lebrini, H. Vezin, F. Chai, M. Traisnel, M. Lagrené, Enhanced corrosion resistance of carbon steel in normal sulfuric acid medium by some macrocyclic polyether compounds containing a 1, 3, 4-thiadiazole moiety: AC impedance and computational studies, Corrosion Science, 2009, 51, 2165-2173.
- H. Ouici, M. Tourabi, O. Benali, C. Selles, C. Jama, A. Zarrouk, F. Bentiss, Adsorption and corrosion inhibition properties of 5-amino 1, 3,
-thiadiazole-2-thiol on the mild steel in hydrochloric acid medium: Thermodynamic, surface and electrochemical studies, Journal of Electroanalytical Chemistry, 2017, 803,125-134.
- M. A. Hegazy, A. S. El-Tabei, A. H. Bedair, M. A. Sadeq, An investigation of three novel nonionic surfactants as corrosion inhibitor for carbon steel in 0.5 M H2SO4, Corrosion Science, 2012, 54, 219-230.
- E. Kowsari, M. Payami, R. Amini, B. Ramezanzadeh, M. Javanbakht, Task-specific ionic liquid as a new green inhibitor of mild steel corrosion, Applied Surface Science, 2014, 289, 478-486.
- M. J. C. Dewar, W. Thiel, Ground states of molecules. 38. The MNDO method. Approximations and parameters, Journal of the American Chemical Society, 1977, 99,
-4907.
DOI: http://dx.doi.org/10.13171/mjc02003171332mm
Refbacks
- There are currently no refbacks.
Copyright (c) 2020 Mediterranean Journal of Chemistry
