Croweacin and Ammi visnaga (L.) Lam Essential Oil derivatives as green corrosion inhibitors for brass in 3% NaCl medium: Quantum Mechanics investigation and Molecular Dynamics Simulation Approaches
Abstract
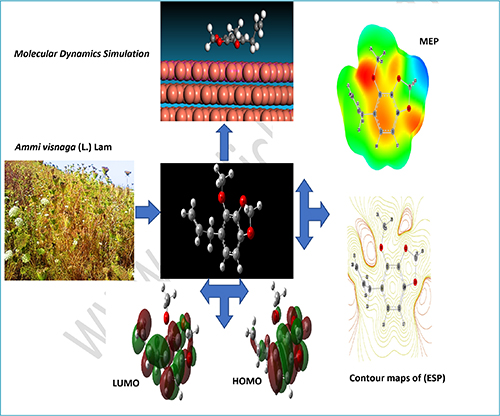
The computational study was carried out to understand the anti-corrosion properties of Croweacin, a major chemical component of two essential oils of Ammi visnaga (L.) Lam collected from northern Morocco in 2016 (EO16) and 2018 (EO18) against brass corrosion in a 3% NaCl medium. The study, moreover, considers the inhibitory effect of some minor compounds of EO18 such as Eugenol, Trans-Anethole, α-Isophorone, and Thymol. In this context, the quantum mechanics modelling using the density functional theory (DFT) method with B3LYP /6-31G (d, p) were conducted in the aqueous medium by the use of the IEFPCM model and SCRF theory. The DFT method was adopted to identify, analyze and interpret several elements such as the electronic features, the Frontier Molecular Orbitals (FMO) diagram, Molecular Electrostatic Potential (MEP), contours maps of the electrostatic potential (ESP), and the Mulliken population analysis. The DFT demonstrated that the studied compounds are excellent corrosion inhibitors.
Furthermore, the Monte Carlo (MC) type simulation of molecular dynamics (MD) was carried out to provide information on the adsorption mechanism of the studied inhibitors through the active sites on the metal surface. This method informed us that the studied inhibitors have high adsorption energy when interacting with the metal surface, especially for Croweacin (-68.63 kcal/mol). The results obtained from DFT and the MC type simulations are in good agreement.
Full Text:
PDFReferences
- S. Aribo, S. J. Olusegun, L. J. Ibhadiyi, A. Oyetunji, D. O. Folorunso, Green inhibitors for corrosion protection in acidizing oilfield environment, Journal of the Association of Arab Universities for Basic and Applied Sciences, 2017, 24, 34-38.
- N. Cao, Y. Miao, D. Zhang, R. Boukherroub, X. Lin, H. Ju, H. Li, Preparation of mussel-inspired perfluorinated polydopamine film on brass substrates: Superhydrophobic and anti-corrosion application, Progress in Organic Coatings, 2018, 125, 109-118.
- S. A. Haddadi, E. Alibakhshi, G. Bahlakeh, B. Ramezanzadeh, M. Mahdavian, A detailed atomic level computational and electrochemical exploration of the Juglans regia green fruit shell extract as a sustainable and highly efficient green corrosion inhibitor for mild steel in 3.5 wt% NaCl solution, Journal of Molecular Liquids, 2019, 284, 682-699.
- H. Cen, Z. Chen, X. Guo, N, S co-doped carbon dots as effective corrosion inhibitor for carbon steel in CO2-saturated 3.5% NaCl solution, Journal of the Taiwan Institute of Chemical Engineers, 2019, 99, 224-238.
- N. B. Seddik, I. Raissouni, K. Draoui, A. A. Aghzzaf, A. Chraka, B. Aznag, F. Chaouket, D. Bouchta, Calcite, the main corrosion inhibitor contained in the raw clay (Rhassoul) of brass in 3% NaCl medium, Mediterranean Journal of Chemistry, 2019, 9, 236-248.
- N. B. Seddik, I. Raissouni, K. Draoui, A. A. Aghzzaf, A. Chraka, B. Aznag, F. Chaouket, D. Bouchta, Anticorrosive performance of lanthanum ions intercalated Stevensite clay on brass in 3% NaCl medium, Materials Today: Proceedings, 2020, 22, 78-82.
- P. Refait, C. Rahal, M. Masmoudi, Corrosion inhibition of copper in 0.5 M NaCl solutions by aqueous and hydrolysis acid extracts of olive leaf. Journal of Electroanalytical Chemistry, 2020, 859, 113834.
- A. Bouoidina, F. El-Hajjaji, A. Abdellaoui, Z. Rais, M. F. Baba, M. Chaouch, O. Karzazi, A. Lahkimi, M. Taleb, Theoretical and Experimental study of the corrosion inhibition of mild steel in acid medium using some surfactants of the essential oil of Foeniculum Vulgare bulb, Journal of Materials and Environmental Sciences, 2017, 8, 1328-1339.
- M. Manssouri, A. Laghchimi, A. Ansari, M. Znini, Z. Lakbaibi, Y. El Ouadi, M. Lhou, Effect of Santolina pectinata (Lag.) Essential Oil to protect against the corrosion of Mild steel in 1.0 M HCl: Experimental and quantum chemical studies, Mediterranean Journal of Chemistry, 2020 , 10, 253-68.
- K. Boumhara, M. Tabyaoui, C. Jama, F. Bentiss, Artemisia Mesatlantica essential oil as green inhibitor for carbon steel corrosion in 1 M HCl solution: Electrochemical and XPS investigations, Journal of Industrial and Engineering Chemistry, 2015, 29, 146-155.
- Y. Ye, D. Yang, H. Chen, A green and effective corrosion inhibitor of functionalized carbon dots, Journal of Materials Science & Technology, 2019, 35, 2243-2253.
- Y. Ye, Z. Jiang, Y. Zou, H. Chen, S. Guo, Q. Yang, L. Chen, Evaluation of the inhibition behavior of carbon dots on carbon steel in HCl and NaCl solutions, Journal of Materials Science & Technology, 2020, 43, 144-153.
- M. Behpour, S. Ghoreishi, N. Soltani, M. Salavati-Niasari, M. Hamadanian, A. Gandomi, Electrochemical and theoretical investigation on the corrosion inhibition of mild steel by thio-salicylaldehyde derivatives in hydrochloric acid solution, Corrosion Science, 2008, 50, 2172-2181.
- F. El-Hajjaji, M. Messali, A. Aljuhani, M. Aouad, B. Hammouti, M. Belghiti, D. Chauhan, M. Quraishi, Pyridazinium-based ionic liquids as novel and green corrosion inhibitors of carbon steel in acid medium: electrochemical and molecular dynamics simulation studies, Journal of Molecular Liquids, 2018, 249, 997-1008.
- E. Alibakhshi, M. Ramezanzadeh, G. Bahlakeh, B. Ramezanzadeh, M. Mahdavian, M. Motamedi, Glycyrrhiza glabra leaves extract as a green corrosion inhibitor for mild steel in 1M hydrochloric acid solution: experimental, molecular dynamics, Monte Carlo and quantum mechanics study, Journal of Molecular Liquids, 2018, 255, 185-198.
- Y. Ye, D. Yang, H. Chen, S. Guo, Q. Yang, L. Chen, H. Zhao, L. Wang, A high-efficiency corrosion inhibitor of N-doped citric acid-based carbon dots for mild steel in hydrochloric acid environment, Journal of hazardous materials, 2020, 381, 121019.
- C. Verma, H. Lgaz, D. Verma, E. E. Ebenso, I. Bahadur, M. Quraishi, Molecular dynamics and Monte Carlo simulations as powerful tools for study of interfacial adsorption behavior of corrosion inhibitors in aqueous phase: a review, Journal of Molecular Liquids, 2018, 260, 99-120.
- S. K. Saha, P. Ghosh, A. Hens, N. C. Murmu, P. Banerjee, Density functional theory and molecular dynamics simulation study on corrosion inhibition performance of mild steel by mercapto-quinoline Schiff base corrosion inhibitor, Physica E: Low-dimensional systems and nanostructures, 2015, 66, 332-341.
- C. Verma, M. A. Quraishi, E. E. Ebenso, I. Bahadur, A Green and Sustainable Approach for Mild Steel Acidic Corrosion Inhibition Using Leaves Extract: Experimental and DFT Studies, Journal of Bio- and Tribo-Corrosion, 2018, 4, 33.
- C. Verma, I. B. Obot, I. Bahadur, E.-S. M. Sherif, E. E. Ebenso, Choline based ionic liquids as sustainable corrosion inhibitors on mild steel surface in acidic medium: Gravimetric, electrochemical, surface morphology, DFT and Monte Carlo simulation studies, Applied Surface Science, 2018, 457, 134-149.
- A. Chraka, I. Raissouni, N. Benseddik, S. Khayar, A. Ibn Mansour, H. Belcadi, F. Chaouket, D. Bouchta, Aging time effect of Ammi visnaga (L.) lam essential oil on the chemical composition and corrosion inhibition of brass in 3% NaCl medium. Experimental and theoretical studies, Materials Today: Proceedings, 2020, 22, 83-88.
- S. A. Haddadi, E. Alibakhshi, G. Bahlakeh, B. Ramezanzadeh, M. Mahdavian, A detailed atomic level computational and electrochemical exploration of the Juglans regia green fruit shell extract as a sustainable and highly efficient green corrosion inhibitor for mild steel in 3.5 wt% NaCl solution, Journal of Molecular Liquids, 2019, 284, 682-699.
- M. Frisch, G. Trucks, H. Schlegel, G. Scuseria, M. Robb, J. Cheeseman, J. Montgomery, T. Vreven, K. Kudin, J. Burant, Gaussian 09, revision B. 01, Gaussian, Inc., Wallingford CT, 2009 Search PubMed;(b) NM O'Boyle, AL Tenderholt and KM Langner, J. Comput. Chem., 2008, 29, 839.
- K. O. Sulaiman, A. T. Onawole, O. Faye, D. T. Shuaib, Understanding the corrosion inhibition of mild steel by selected green compounds using chemical quantum-based assessments and molecular dynamics simulations, Journal of Molecular Liquids, 2019, 279, 342-350.
- J. Tomasi, B. Mennucci, R. Cammi, Quantum mechanical continuum solvation models, Chemical reviews, 2005, 105, 2999-3094.
- A. Frisch, A. Nielson, A. Holder, Gaussview user manual, Gaussian Inc., Pittsburgh, PA, 2000, 556.
- B. Gomez, N. Likhanova, M. Dominguez-Aguilar, R. Martinez-Palou, A. Vela, J. L. Gazquez, Quantum chemical study of the inhibitive properties of 2-pyridyl-azoles, The Journal of Physical Chemistry B, 2006, 110, 8928-8934.
- R. G. Pearson, Absolute electronegativity and hardness: application to inorganic chemistry, Inorganic chemistry, 1988, 27, 734-740.
- D. Frenkel, B. Smit, Understanding molecular simulation: From algorithms to applications, Elsevier, 2002, 1, 1-638.
- N. Kovačević, I. Milošev, A. Kokalj, The roles of mercapto, benzene, and methyl groups in the corrosion inhibition of imidazoles on copper: II. Inhibitor–copper bonding, Corrosion Science, 2015, 98, 457-470.
- S. Kaya, B. Tüzün, C. Kaya, I. B. Obot, Determination of corrosion inhibition effects of amino acids: quantum chemical and molecular dynamic simulation study, Journal of the Taiwan Institute of Chemical Engineers, 2016, 58, 528-535.
- H. Sun, P. Ren, J. Fried, The COMPASS force field: parameterization and validation for phosphazenes, Computational and Theoretical Polymer Science, 1998, 8, 229-246.
- H. Sun, COMPASS: an ab initio force-field optimized for condensed-phase applications overview with details on alkane and benzene compounds, The Journal of Physical Chemistry B, 1998, 102, 7338-7364.
- H. C. Andersen, Molecular dynamics simulations at constant pressure and/or temperature, The Journal of chemical physics, 1980, 72, 2384-2393.
- S. John, J. Joy, M. Prajila, A. Joseph, Electrochemical, quantum chemical, and molecular dynamics studies on the interaction of 4‐amino‐4H, 3, 5‐di (methoxy)‐1, 2, 4‐triazole (ATD), BATD, and DBATD on copper metal in 1N H2SO4, Materials and Corrosion, 2011, 62, 1031-1041.
- N. Asadi, M. Ramezanzadeh, G. Bahlakeh, B. Ramezanzadeh, Utilizing Lemon Balm extract as an effective green corrosion inhibitor for mild steel in 1M HCl solution: a detailed experimental, molecular dynamics, Monte Carlo and quantum mechanics study, Journal of the Taiwan Institute of Chemical Engineers, 2019, 95, 252-272.
- D. K. Yadav, B. Maiti, M. Quraishi, Electrochemical and quantum chemical studies of 3, 4-dihydropyrimidin-2 (1H)-ones as corrosion inhibitors for mild steel in hydrochloric acid solution, Corrosion Science, 2010, 52, 3586-3598.
- M. Masoud, M. Awad, M. Shaker, M. El-Tahawy, The role of structural chemistry in the inhibitive performance of some amino pyrimidines on the corrosion of steel, Corrosion Science, 2010, 52, 2387-2396.
- G. Gao, C. Liang, Electrochemical and DFT studies of β-amino-alcohols as corrosion inhibitors for brass, Electrochimica Acta, 2007, 52, 4554-4559.
- H. Tanak, A. Ağar, M. Yavuz, Experimental and quantum chemical calculational studies on 2-[(4-Fluorophenylimino) methyl]-3, 5-dimethoxyphenol, Journal of molecular modeling, 2010, 16, 577-587.
- R. G. Parr, R. G. Pearson, Absolute hardness: companion parameter to absolute electronegativity, Journal of the American Chemical Society, 1983, 105, 7512-7516.
- O. Benali, L. Larabi, M. Traisnel, L. Gengembre, Y. Harek, Electrochemical, theoretical and XPS studies of 2-mercapto-1-methylimidazole adsorption on carbon steel in 1 M HClO4, Applied surface science, 2007, 253, 6130-6139.
- M. Ameer, A. Fekry, Corrosion inhibition of mild steel by natural product compound, Progress in Organic Coatings, 2011, 71, 343-349.
- K. Anupama, A. Joseph, Experimental and theoretical studies on Cinnamomum verum leaf extract and one of its major components, eugenol as environmentally benign corrosion inhibitors for mild steel in acid media, Journal of Bio-and Tribo-Corrosion, 2018, 4, 30.
- L. O. Olasunkanmi, I. B. Obot, M. M. Kabanda, E. E. Ebenso, Some quinoxalin-6-yl derivatives as corrosion inhibitors for mild steel in hydrochloric acid: experimental and theoretical studies, The Journal of Physical Chemistry C, 2015, 119, 16004-16019.
- F. El Hajjaji, H. Greche, M. Taleb, A. Chetouani, A. Aouniti, B. Hammouti, Application of essential oil of thyme vulgaris as green corrosion inhibitor for mild steel in1M HCl, J Mater Environ Sci., 2016, 7, 566-578.
- A. Mishra, C. Verma, H. Lgaz, V. Srivastava, M. Quraishi, E. E. Ebenso, Synthesis, characterization and corrosion inhibition studies of N-phenyl-benzamides on the acidic corrosion of mild steel: Experimental and computational studies, Journal of Molecular Liquids, 2018, 251, 317-332.
- V. Sastri, J. Perumareddi, Molecular orbital theoretical studies of some organic corrosion inhibitors, Corrosion, 1997, 53, 617-622.
- L. C. Murulana, A. K. Singh, S. K. Shukla, M. M. Kabanda, E. E. Ebenso, Experimental and quantum chemical studies of some bis (trifluoromethyl-sulfonyl) imide imidazolium-based ionic liquids as corrosion inhibitors for mild steel in hydrochloric acid solution, Industrial & Engineering Chemistry Research, 2012, 51, 13282-13299.
- M. Belghiti, S. Echihi, A. Dafali, Y. Karzazi, M. Bakasse, H. Elalaoui-Elabdallaoui, L. Olasunkanmi, E. Ebenso, M. Tabyaoui, Computational simulation and statistical analysis on the relationship between corrosion inhibition efficiency and molecular structure of some hydrazine derivatives in phosphoric acid on mild steel surface, Applied Surface Science, 2019, 491, 707-722.
- L. H. Madkour, S. Kaya, C. Kaya, L. Guo, Quantum chemical calculations, molecular dynamics simulation and experimental studies of using some azo dyes as corrosion inhibitors for iron. Part 1: Mono-azo dye derivatives, Journal of the Taiwan Institute of Chemical Engineers, 2016, 68, 461-480.
- I. Obot, Z. Gasem, Theoretical evaluation of corrosion inhibition performance of some pyrazine derivatives, Corrosion Science, 2014, 83, 359-366.
- A. Singh, K. R. Ansari, M. A. Quraishi, Y. Lin, Investigation of Corrosion Inhibitors Adsorption on Metals Using Density Functional Theory and Molecular Dynamics Simulation, in Corrosion Inhibitors, IntechOpen, 2019.
- C. Verma, M. Quraishi, E. Ebenso, I. Obot, A. El Assyry, 3-Amino alkylated indoles as corrosion inhibitors for mild steel in 1M HCl: Experimental and theoretical studies, Journal of Molecular Liquids, 2016, 219, 647-660.
- I. Lukovits, E. Kalman, F. Zucchi, Corrosion inhibitors-correlation between electronic structure and efficiency, Corrosion, 2001, 57, 3-8.
- A. Görling, Density-functional theory beyond the Hohenberg-Kohn theorem, Physical Review A, 1999, 59, 3359.
- Y. Karzazi, M. Belghiti, F. El-Hajjaji, B. Hammouti, Density functional theory modeling and monte Carlo simulation assessment of N-substituted quinoxaline derivatives as mild steel corrosion inhibitors in acidic medium, J Mater Environ Sci., 2016, 7, 3916-3929.
- R. Rahmani, N. Boukabcha, A. Chouaih, F. Hamzaoui, S. Goumri-Said, On the molecular structure, vibrational spectra, HOMO-LUMO, molecular electrostatic potential, UV–Vis, first order hyperpolarizability, and thermodynamic investigations of 3-(4-chlorophenyl)-1-(1yridine-3-yl) prop-2-en-1-one by quantum chemistry calculations, Journal of Molecular Structure, 2018, 1155, 484-495.
- P. Mourya, P. Singh, A. Tewari, R. Rastogi, M. Singh, Relationship between structure and inhibition behaviour of quinolinium salts for mild steel corrosion: Experimental and theoretical approach, Corrosion Science, 2015, 95, 71-87.
- R. Hsissou, S. Abbout, A. Berisha, M. Berradi, M. Assouag, N. Hajjaji, A. Elharfi, Experimental, DFT and molecular dynamics simulation on the inhibition performance of the DGDCBA epoxy polymer against the corrosion of the E24 carbon steel in 1.0 M HCl solution, Journal of Molecular Structure, 2019, 1182, 340-351.
- M. Özcan, I. Dehri, M. Erbil, Organic sulphur-containing compounds as corrosion inhibitors for mild steel in acidic media: correlation between inhibition efficiency and chemical structure, Applied surface science, 2004, 236, 155-164.
- O. Dagdag, Z. Safi, H. Erramli, N. Wazzan, L. Guo, C. Verma, E. Ebenso, S. Kaya, A. El Harfi, Epoxy prepolymer as a novel anti-corrosive material for carbon steel in acidic solution: Electrochemical, surface and computational studies, Materials Today Communications, 2020, 22, 100800.
- S. Xia, M. Qiu, L. Yu, F. Liu, H. Zhao,, Molecular dynamics and density functional theory study on relationship between structure of imidazoline derivatives and inhibition performance, Corrosion Science, 2008, 50, 2021-2029.
- E. Alibakhshi, M. Ramezanzadeh, S. Haddadi, G. Bahlakeh, B. Ramezanzadeh, M. Mahdavian, Persian Liquorice extract as a highly efficient sustainable corrosion inhibitor for mild steel in sodium chloride solution, Journal of cleaner production, 2019, 210, 660-672.
- M. K. Awad, M. R. Mustafa, M. M. A. Elnga, Computational simulation of the molecular structure of some triazoles as inhibitors for the corrosion of metal surface, Journal of molecular structure: theochem, 2010, 959, 66-74.
- S. K. Saha, P. Banerjee, A theoretical approach to understand the inhibition mechanism of steel corrosion with two aminobenzonitrile inhibitors, RSC Advances, 2015, 5, 71120-71130.
DOI: http://dx.doi.org/10.13171/mjc10402004281338ac
Refbacks
- There are currently no refbacks.
Copyright (c) 2020 Mediterranean Journal of Chemistry
