Tin corrosion inhibition by molybdate ions in 0.2 M maleic acid solution: Electrochemical and surface analytical study
Abstract
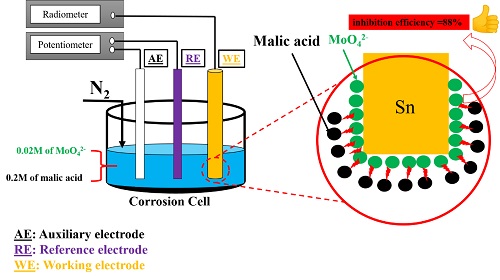
The inhibition efficiency of molybdate ions (MoO42-) against tin corrosion in 0.2 M Malic acid has been studied using electrochemical (DC and AC) and surface analytical methods (SEM and EDX). The electrochemical polarization curves revealed the presence of an active/passive transition of the tin electrode. The electrochemical impedance measurements (EIS) confirmed the benefic effect of increasing MoO42- concentration on the inhibition efficiency (η %) (reaching ηmax ≈ 88% at 0.02 M), whereas η % decreases by increasing temperature. The molybdate ions inhibition mechanism was attributed to the adsorption on the metal surface involving the formation of the adsorbed protective layer.
Full Text:
PDFReferences
- D. Xia, S. Song, W. Gong, Y. Jiang, Z. Gao, J. Wang, Detection of corrosion-induced metal release from tinplate cans using a novel electrochemical sensor and inductively coupled plasma mass spectrometer, J. Food Eng., 2012, 113, 11–18.
- T. Benabbouha, M. Siniti, H. El Attari, K. Chefra, F. Chibi, R. Nmila, H. Rchid, Red Algae Halopitys Incurvus Extract as a Green Corrosion Inhibitor of Carbon Steel in Hydrochloric Acid, J Bio TriboCorros., 2018, 4, 39.
- R. B. Channouf, N. Souissi, S. Zanna, H. Ardelean, N. Bellakhal, P. Marcus, Surface Characterization of the Corrosion Product Layer Formed on Synthetic Bronze in Aqueous Chloride Solution and the Effect of the Adding of Juniperus Communis Extract by X-Ray Photoelectron Spectroscopy Analysis, Chemistry Africa, 2018, 1(3–4), 167–174.
- S. C. Nwanonenyi, H. C. Obasi, I. C. Chukwujike, M. A. Chidiebere, E. E. Oguzie, Inhibition of Carbon Steel Corrosion in 1 M H2SO4 Using Soy Polymer and Polyvinylpyrrolidone, Chemistry Africa, 2019, 2, 277–289.
- M. Dudukcu, B. Yazici, M. Erbil, The effect of indole on the corrosion behaviour of stainless steel, Mater Chem Phys, 2004, 87, 138-141.
¬¬6- A. Galal, N. F. Atta, Al-Hassan MHS Mater, Effect of some thiophene derivatives on the electrochemical behavior of AISI 316 austenitic stainless steel in acidic solutions containing chloride ions: I. Molecular structure and inhibition efficiency relationship, Chem Phys, 2005, 89, 38-48.
- S. A. Umoren, M. M. Solomon, I. B. Obot, R. K. Sulieman, A critical review on the recent studies on plant biomaterials as corrosion inhibitors for industrial metals, J Ind Eng Chem, 2019, 76, 91-115.
- A. C. Clark, P. D. Prenzler, Impact of the condition of storage of tartaric acid solutions on the production and stability of glyoxylic acid, Scollary, Food Chem, 2007, 102, 905-916.
- C. Y. Chan, K. H. Khoo, Y. C. Chua, S. Br. Guruswamy, Potentiodynamic studies of tin corrosion in presence of citrate and bisulphate ions in aqueous solutions of varying pH, British Corros J, 1993, 28, 53-58.
- H. Bensabra, A. Franczak, O. Aaboubi, Inhibitive effect of molybdate ions on the electrochemical behavior of steel rebar in simulated concrete pore solution, Metall and Mat Trans A, 2017, 48, 412-424.
- Y. Ait Albrimi, A. Ait Addi, J. Douch, R. M. Souto, M. Hamdani, Inhibition of the pitting corrosion of 304 stainless steel in 0.5 M hydrochloric acid solution by heptamolybdate ions, Corrosion Sci., 2015, 90, 522–528.
- S. Ramesh, S. Rajeswari, Corrosion inhibition of mild steel in neutral aqueous solution by new triazole derivatives, Electrochim Acta, 2004, 49, 811–820.
- M. Saremi, C. Dehghanian, M. M. Sabet, The effect of molybdate concentration and hydrodynamic effect on mild steel corrosion inhibition in simulated cooling water, Corros Sci, 2006, 48, 1404–1412.
- F. Eghbali, M. H. Moayed, A. Davoodi, N. Ebrahimi, Critical pitting temperature (CPT) assessment of 2205 duplex stainless steel in 0.1 M NaCl at various molybdate concentrations, Corros Sci, 2011, 53, 513–522.
- E. Brahim, J. Aaziz, E. Khadija, B. Ali, E. Souad, B. Lahcen, H. Mustapha, Effect of solution's pH and molecular structure of three linear a-amino acids on the corrosion of tin in salt solution: A combined experimental and theoretical approach, Journal of Molecular Structure, 2019, 1196, 105-118.
- E. Brahim, J. Aaziz, E. Khadija, B. Ali, E. Souad, B. Lahcen, H. Mustapha, Understanding the influence of solution's pH on the corrosion of tin in saline solution containing functional amino acids using electrochemical techniques and molecular modeling, Surfaces and Interfaces, 2019, 17, 100-343.
- F. Eghbali, M. H. Moayed, A. Davoodi, N. Ebrahimi, Critical pitting temperature (CPT) assessment of 2205 duplex stainless steel in 0.1 M NaCl at various molybdate concentrations, Corros Sci, 2011, 53, 513–522.
- S.A.M. Refaey, Inhibition of steel pitting corrosion in HCl by some inorganic anions, Appl Surf Sci, 2005, 240, 396–404.
- G. Mu, X. Li, Q. Qu, J. Zhou, Molybdate and tungstate as corrosion inhibitors of steel in hydrochloric acid solution, Corrosion Science, 2006, 48, 445-459.
- S. S. Abd El-Rehim, S. A. M. Refaey, F. Taha, M. B. Saleh, R. A. Ahmed, Corrosion Inhibition of Mild Steel in Acidic Medium using 2-amino Thiophenoland 2-Cyanomethyl Benzothiazole, J Appl Electrochem., 2001, 31, 429–435.
- M. Lee, S. Sohn, M. Lee, Ionic equilibria and ion exchange of molybdenum(VI) from strong acid solution, Bull Korean Chem Soc, 2011, 32, 3687-3691.
- E. Fujioka, H. Nishihara, K. Aramaki, The inhibition of pit nucleation and growth on the passive surface of iron in a borate buffer solution containing Cl− by oxidizing inhibitors, Corros. Sci., 1996, 38, 1915-1933.
- S. A. M. Refaey, S. S. Abd El-Rehim, F. Taha, M. B. Saleh, R. A. Ahmed, Inhibition of chloride localized corrosion of mild steel by PO43−, CrO42−, MoO42−, and NO2− anions, Applied Surface Science, 2000, 158, 190–196.
- M. A. Deyab, S. S. Abd EI-Rehim, Inhibitory effect of tungstate, molybdate and nitrite ions on the carbon steel pitting corrosion in alkaline formation water containing Cl− ion, Electrochim. Acta, 2007, 53, 1754–1760.
- E. H. Ait Addi, L. Bazzi, M. Elhilali, R. Salghi, B. Hammouti, M. Mihitb, Effect of the addition of oxo-anions on the corrosion and passivation of tin in synthetic industrial water, Applied Surface Science, 2006, 253, 555-560.
- A. Ait Addi, E. H. Ait Addi, I. Bakas, M. Hamdani, The Effect of Molybdate Anions on the Corrosion and Passivation of Tinplate in Synthetic Industrial, Water Int J Electrochem Sci, 2014, 9, 8465-8475.
- G. O. Ilevbare, G. T. Burstein, The inhibition of pitting corrosion of stainless steels by chromate and molybdate ions, Corros. Sci., 2003, 45, 1545–1569.
- C. O. Akalezi, G. O. Onyedika, H. F. Chahul, E. E. Oguzie, Experimental and theoretical studies on the corrosion inhibition of mild steel in acidic media by Pentaclethra macrophylla plant extract, FUTOJNLS, 2016, 2(1), 265-280.
- S. C. Nwanonenyi, O. Ogbobe, E. E. Oguzie, Protection of Mild Steel Corrosion in Sulphuric Acid Environment Using Wheat Starch, International Journal of Engineering and Technologies, 2017, 10, 11-21.
- M. S. Vukasovich, J. P. G. Farr, Molybdate in corrosion inhibition- A review. Polyhedron., 1986, 5(1-2), 551-559.
- G. Wilcox, D. Gabe, M. Warwick, The role of molybdates in corrosion prevention. Corros. Rev, 1986, 6, 328-365.
- I. L. Rozenfeld, Corrosion Inhibitors, McGraw Hill, New York, 1981, 97.
- J. G. M. Thomas, Corrosion, third ed., Butterworth Heinemann, Oxford, 1994, 17, 40–17:65.
- S. C. Nwanonenyi, H. C. Obasi, I. O. Eze, Hydroxypropyl Cellulose as an Efcient Corrosion Inhibitor for Aluminium in Acidic Environments: Experimental and Theoretical Approach, Chemistry Africa, 2019, 2, 471–482.
- I. Carrillo, B. Valdez, R. Zlatev, M. Stoytcheva, M. Carrillo, R. Bäßler, Electrochemical study of oxyanions effect on galvanic corrosion inhibition, Int. J. Electrochem. Sci., 2012, 7, 8688-8701.
- A. I. Onuchukwu, A. I. Baba, A study of the effects of ionogen on the corrosion stripping of the ZN surface of galvaiized steel in an aqueous medium, Mater Chem Phys, 1987, 18(4),
–390.
- S. Alshamsi Ahmed, A. AlBlooshi, Effect of Surface Roughness on the Corrosion Behavior of Pure Iron in Acidic Solutions, Int. J. Electrochem. Sci., 2019, 14, 5794-5812.
- O. L. Riggs, Jr., Theoretical aspects of corrosion inhibitors and inhibition, Corrosion Inhibition, 1973, second ed., C.C. Nathan, Houston, TX.
- H. Lgaz, K. S. Bhat, R. Salghi, S. Jodeh, M. Algarra, B. Hammouti, IH. Ali, A. Essamri, Insights into corrosion inhibition behavior of three chalcone derivatives for mild steel in hydrochloric acid solution, J Mol Liq., 2017, 238, 71–83.
- M. Elayyachy, A. El Idrissi, B. Hammouti, New thio-compounds as corrosion inhibitor for steel in 1M HCl, Corros. Sci., 2006, 48, 2470-2479.
- S. M. Rezaei Niya, M. Hoorfar, Electrochim Acta., 2016, 188, 98-02.
- S. Gudic´, I. Smoljko, M. Kliskic´, The effect of small addition of tin and indium on the corrosion behavior of aluminium in chloride solution, Journal of Alloys and Compounds, 2010, 505, 54-63.
- P. Bommersbach, C. Alemany-Dumont, J. P. Millet, B. Normand, Formation and behaviour study of an environment-friendly corrosion inhibitor by electrochemical methods, Electrochim Acta., 2005, 51, 1076-1084.
- F. Bentiss, M. Lebrini, M. Lagrenée, Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in mild steel/2, 5-bis (n-thienyl)-1, 3, 4-thiadiazoles/hydrochloric acid system, Corros. Sci., 2005, 47, 2915-2931.
- S. C. Nwanonenyi, H. C. Obasi, E. E. Oguzie, I. C. Chukwujike, C. K. Anyiam, Inhibition and adsorption of polyvinyl acetate (PVAc) on the corrosion of aluminium in sulphuric and hydrochloric acid environment. J Bio TriboCorros., 2017, 3, 53.
- M. Behpour, S. M. Ghoreishi, N. Soltani, M. Salavati-Niasari, M. Hamadanian, A. Gandomi, Electrochemical and theoretical investigation on the corrosion inhibition of mild steel by thiosalicylaldehyde derivatives in hydrochloric acid solution, Corros. Sci., 2008, 50, 2172-2181.
- J. Aljourani, K. Raeissi, M. A. Golozar, Benzimidazole and its derivatives as corrosion inhibitors for mild steel in 1M HCl solution, Corros. Sci., 2009, 51, 1836-1843.
- O. Radovici, Proc.7th European Symposium on Corrosion Inhibitors, 1990, Ann. Univ. Ferrara, 330, Italy.
- A. I. Onuchukwu, A. I. Baba, a study of the effects of ionogen on the corrosion stripping of the ZN surface of galvanized steel in an aqueous medium, Mater Chem Phys., 1987, 18, 381–390.
DOI: http://dx.doi.org/10.13171/mjc10502005141394aa
Refbacks
- There are currently no refbacks.
Copyright (c) 2020 Mediterranean Journal of Chemistry
