New strategy of synthesis, characterization, theoretical study and inhibition effect on mild steel corrosion in acidic solution
Abstract
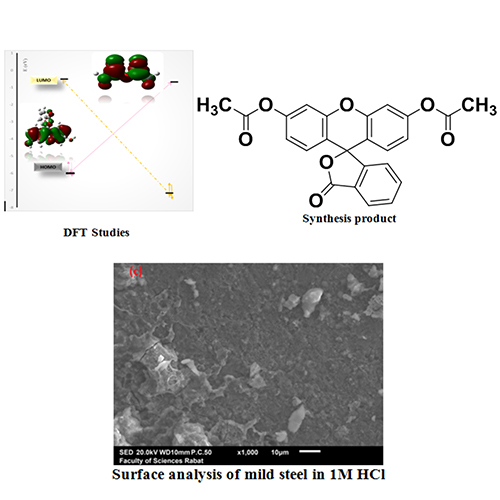
We report two new different synthesis strategies of an eco-friendly organic dye 3-oxo-3H-spiro[isobenzofuran-1,9'-xanthene]-3',6'-diyl diacetate. These methods were based on the reaction of fluorescein with acetic anhydride under different experimental conditions. The synthesized heterocyclic system obtained with excellent yield was characterized by FT-IR, 1H N.M.R., 13C N.M.R., and mass spectrometry. This reaction has also been studied theoretically using Gaussian 09 based on the DFT method at B3LYP/6-31G (d, p). The results obtained are in good correlation with those obtained experimentally. The inhibition behavior of the synthetic product has been studied using absorbance difference measurement, the percentage of inhibition efficiency attains 90% at 10-4M of NAR3 inhibitor. The surface analysis of mild steel was investigated using scanning electron microscopy (S.E.M.) and Energy Dispersive X-ray (E.D.X.) methods and show the adsorption behavior of inhibitor on the surface of mild steel.
Full Text:
PDFReferences
- A. Kumari, R. K. Singh, Medicinal chemistry of indole derivatives: Current to future therapeutic prospectives, Bioorg. Chem, 2019, 89, 103021.
- R. Kharb, P. C. Sharma, M. S. Yar, Pharmacological significance of triazole scaffold, J. Enzyme Inhib. Med. Chem, 2011, 26, 1–21.
- A. Kashyap, O. Silakari, Triazoles: Multidimensional 5‐membered nucleus for designing multitargeting agents. In Key Heterocycle Cores for Designing Multitargeting Molecules; Elsevier: Amsterdam. Netherlands, 2018, 323–342.
- C. V. Maftei, E. Fodor, P. G. Jones, C. G. Daniliuc, M. H. Franz, G. Kelter, H. Fiebig, M. Tamm, I. Neda, Novel 1.2.4‐oxadiazoles and trifluoromethylpyridines related to natural products: Synthesis. structural analysis and investigation of their antitumor activity, Tetrahedron, 2016, 72, 1185–1199.
- I. Neda, T. Kaukorat, R. Schmutzler, U. Niemeyer, B. Kutscher, J. Pohl, J.Engel, Benzodiaza‐, Benzoxaza‐ and benzodioxa-phosphorinones‐formation, reactivity, structure and biological activity, Phosphorus Sulfur Silicon 2000, 162, 81–218.
- M. Simon, C. sunderlik, L. Cotarca, M. T. Caproiu, I. Neda, M. C. Turoczi, R. Volpicelli, Synthesis of new active 0‐nitrophenyl carbamates, Synth. Comun., 2005, 35, 1471–1479.
- Y. Mohammad, K. M. Fazili, K. A. Bhat, T. Ara, Synthesis and biological evaluation of novel 3‐O‐tethered triazoles of diosgenin as potent antiproliferative agents, Steroids, 2017, 118, 1–8.
- M. Huang, Z. Deng, J. Tian, T. Liu, Synthesis and biological evaluation of salinomycin triazole analogues as anticancer agents, Eur. J. Med. Chem., 2017, 127, 900‐908.
- R. Gujjar, A. Marwaha, F. El Mazouni, J. White, K. L. White, S. Creason, D. M. Shackleford, J. Baldwin, W. N. Charman, F. S. Buckner, Identification of a metabolically stable triazolo-pyrimidine‐based dihydroorotate dehydrogenase inhibitor with antimalarial activity in mice, J. Med. Chem., 2009, 52, 1864–1872.
- M. Chen, S. Lu. G. Yuan, S. Yang, X. Du, Synthesis and antibacterial activity of some heterocyclic β‐enamino ester derivatives with 1. 2. 3‐triazole, Heterocycl. Commun., 2000, 6, 421–426.
- A. Ayati, S. Emami, A. Foroumadi, The importance of triazole scaffold in the development of anticonvulsant agents, Eur. J. Med. Chem., 2016, 109, 380–392.
- T. Akhtar, S. Hameed, K. M. Khan, M. I. Choudhary, Syntheses, urease inhibition, and antimicrobial studies of some chiral 3‐substituted‐4‐amino‐5‐thioxo‐1H, 4H‐1.2.4‐triazoles, Med. Chem., 2008, 4,
–543.
- L. Sevaille, L. Gavara, C. Bebrone, F. De Luca, L. Nauton, M. Achard, P. Mercuri, S. Tanfoni, L. Borgianni, C. Guyon, 1.2.4‐Triazole‐3‐thione compounds as inhibitors of dizinc metallo‐β‐ lactamases, Chem Med Chem., 2017, 12, 972–985.
- P. Raffaello, Mono- and Di-Quaternized 4,4’-Bipyridine Derivatives as Key Building Blocks for Medium- and Environment-Responsive Compounds and Materials, Molecules, 2020, 25, 1-30.
- S. Achamlale, E. H. Mabrouk, A. Elachqar, A. El Hallaoui, S. El Hajji, A. Alami, J. Bellan, M. R. Mazières, J. G. Wolf, M. Pierrot, Synthesis and Thermal Isomerization of Carboxylic and Phosphonic α-Aminoesters Substituted with a Triazole Ring, Phosphorus, Sulfur, and Silicon and the Related Elements, 2007, 182, 357-367.
- E. H. Mabrouk, A. Elachqar, A. Alami, A. El Hallaoui, S. El Hajji, One-pot regioselective synthesis of n-benzoyl 2-amino-3,4-dihydro-3-oxo-2h-1,4-benzothiazines, Oriental Journal of Chemistry, 2010, 26(4), 1249-1255.
- E. H. Mabrouk, A. Elachqar, A. El Hallaoui, A. Alami, S. El Hajji, J. Martinez, V. Rolland, Synthesis of new racemic α-heterocyclic α,α-diaminoesters and α-aminoester carboxylic, Arabian Journal of Chemistry, 2013, 6, 93-96.
- L. Lingjun, L. Jiyuan, D. Wenhao, G. Feng, C. Kaixian, Z. Yu, L. Hong, Rhodium(III)-Catalyzed Redox-Neutral [3+3] Annulation of N-nitrosoanilines with Cyclopropenones: A Traceless Approach to Quinolin-4(1H)-One Scaffolds, Molecules, 2020, 25, 268-282.
- L. Mousa, R. M. Al-Smadi, M. Amjad, F. K. Omar, M. M. Majed, H. A. Karem, Synthesis, Characterization, Antimicrobial Activity, and Genotoxicity Assessment of Two Heterocyclic Compounds Containing 1.2.3-Selena- or 1.2.3-Thiadiazole Rings, Molecules, 2019, 24,
-4092.
- S. N. Sirakanyan, E. K. Akopyan, R. G. Paronikyan, A. G. Akopyan, A. A. Ovakimyan, Synthesis and anticonvulsant activity of 7(8)-amino derivatives of condensed thieno[3.2-d] pyrimidines, Pharm. Chem. J., 2016, 50, 296–300.
- S. N. Sirakanyan, A. Geronikaki, D. Spinelli, E. K. Hakobyan, V. G. Kartsev, A. Petrou, A. A. Hovakimyan, Synthesis and antimicrobial activity of new amino derivatives of pyrano[4”.3”:40 .50]pyrido[30.20:4.5] thieno[3.2-d]pyrimidine, An. Acad. Bras. Cienc., 2018, 90, 1043–1057.
- S. N. Sirakanyan, D. Spinelli, A. Geronikaki, V. G. Kartsev, E. K. Hakobyan, A. A Hovakimyan,. Synthesis and antimicrobial activity of new derivatives of pyrano-[4”.3”:40.50]pyrido[30.20 :4.5]thieno[3.2-d]pyrimidine and new heterocyclic systems, Synth. Commun., 2019, 49, 1262–1276.
- N. S. Samvel, S. Domenico, G. Athina, K. H. Elmira, S. Harutyun, A. Erik, Z. Hovakim, E. N. Lusine, S. A. Anahit, S. D. Irina, E. M. Rafayel, A. H. Anush, Synthesis, Antitumor Activity, and Docking Analysis of New Pyrido-[30,20 :4,5]furo(thieno)[3,2-d]pyrimidin-8-amines, Molecules, 2019, 24, 3952-3965.
- S. Cascioferro, B. Parrino, V. Spanò, A. Carbone, A. Montalbano, P. Barraja, P. Diana, G. Cirrincione, An overview on the recent developments of 1,2,4-triazine derivatives as anticancer compounds, Eur. J. Med. Chem., 2017, 142, 328–375.
- M. Sztanke, K. Sztanke, B. Rajtar, Ł. Swiatek, A. Boguszewska, M. Polz-Dacewicz, the influence of some promising fused azaiso-cytosine-containing congeners on zebrafish (Danio rerio) embryos/larvae and their antihaemolytic, antitumour and antiviral activities, Eur. J. Pharm. Sci., 2019, 132, 34–43.
- P. M. S Monk, The Viologens: Physicochemical Properties, Synthesis and Applications of the Salts of 4,4-Bipyridine; Chichester; New York: Wiley, 1998.
- M. Baroncini, S. Silvi, A. Credi, Photo- and Redox-Driven Artificial Molecular Motors, Chem. Rev., 2020, 120, 1, 200-268.
- Y. Wang, M. Frasconi, J. F. Stoddart, Introducing Stable Radicals into Molecular Machines, A.C.S. Cent. Sci., 2017, 3, 927–935.
- S. Erbas-Cakmak, D. A. Leigh, C. T. McTernan, A. L. Nussbaumer, Artificial Molecular Machines, Chem. Rev., 2015, 115, 10081–10206.
- A. C. Fahrenbach, Z. Zhu. D. Cao, W. G. Liu, H. Li, S. K. Dey, S. Basu, A. Trabolsi, Y. Y. Botros, Goddard III. W. A, J. F. Stoddart, Radically Enhanced Molecular Switches, J. Am. Chem. Soc., 2012, 134, 16275–16288.
- B. L. Feringa, W. R. Browne, Molecular Switches; Wiley V.C.H.: Weinheim, Germany, 2011, 2, pp. 792, ISBN 978-3-527-63441-5.
- V. Buchmann, K. D. Weston, M. Sauer, Spectroscopic study and evaluation of redabsorbing fluorescent dyes, Bioconjugate Chem., 2003, 14, 195-204.
- G. Cosa, K. S. Focsaneanu, J. R. N. Mclean, J. P. McNamee, J. C. Scaiano, Photophysical properties of fluorescent DNA-dyes bound to single- and double-stranded D.N.A. in aqueous buffered solution, Photochem. Photobiol., 2001, 73, 585-599.
- E. V. R. de Castro, F. E. Jorge, Accurate universal Gaussian basis set for all atoms of the periodic table, Journal of Chemical Physics, 199, 108, 5225-5229.
- N. Chafai, S. Chafaa, K. Benbouguerra, D. Daoud, A. Hellal, M. Mehri, Synthesis, characterization and the inhibition activity of a new α-aminophosphonic derivative on the corrosion of XC48 carbon steel in 0.5 M H2SO4: Experimental and theoretical studies, Journal of the Taiwan Institute of Chemical Engineers, 2017, 70, 331-344.
- A. Hellal, S. Chafaa, N. Chafai, L. Touafri, Synthesis, antibacterial screening and DFT studies of series of α-amino-phosphonates derivatives from aminophenols, Journal of Molecular Structure, 2017, 1134, 217-225.
- L. R. Domingo, P. Pérez, The nucleophilicity N index in organic chemistry, Organic and Biomolecular Chemistry, 2011, 9, 7168-7175.
- P. Pérez, L. R. Domingo, M. Duque-Noreňa, E. A. Chamorro, Condensed-to-atom nucleophilicity index. An application to the director effects on the electrophilic aromatic substitutions, Journal of Molecular Structure (Theochem), 2009, 895, 86-91.
- H. Eshghi, N. Mirzaie, A. Asoodeh, Dyes and Pigments Synthesis of fluorescein aromatic esters in the presence of P2O5/SiO2 as catalyst and their hydrolysis studies in the presence of lipase, Dye Pigment, 2011, 89, 120–126.
- A. I. Onen, B. T. Nwufo, E. Ebenso, R. M. Hlophe , Titanium (IV) Oxide as Corrosion Inhibitor for Aluminium and Mild Steel in Acidic Medium, International Journal of Electrochemical Science, 2010, 5, 1563 – 1573.
- A. Ezeibe, E. Nleonu, A. Ahumonye, Thermodynamics Study of Inhibitory Action of Lignin Extract from Gmelina arborea on the corrosion of mild steel in dilute Hydrochloric Acid, International Journal of Scientific Engineering and Research, 2019, 7(2), 133-136.
- F. El-Hajjaji, M. Messali, M. V. Martínez de Yuso, E. Rodríguez-Castellón, S. Almutairi, Teresa J. Bandosz, M. Algarra, Journal of Colloid and Interface Science, 2019, 541, 418–424.
- F. El-Hajjaji, M. Messali, A. Aljuhani, M. R. Aouad, B. Hammouti, M. E. Belghiti, D. S. Chauhan, M. A. Quraishi, Journal of Molecular Liquids, 2019, 249, 997–1008.
- R. Salim, E. Ech-chihbi, H. Oudda, F. El Hajjaji, M. Taleb, S. Jodeh. A review on the assessment of Imidazo[1,2-a] pyridines As corrosion inhibitor of metals, J Bio Tribo Corros, 2019, 5,14.
- F. El Hajjaji, F. Abrigach, O. Hamed, A. R. Hasan, M. Taleb, S. Jodeh, E. Rodríguez-Castellón, M. V. M. Yuso, M. Algarra, Corrosion resistance of mild steel coated with organic material containing pyrazole moiety, Coatings, 2018, 8, 330.
- N. Arshad, A. K. Singh, M. Akram, F. Perveen, I. Rasheed, F. Altaf, P. A. Channar, A. Saeed, Experimental, theoretical, and surface study for corrosion inhibition of mild steel in 1 M HCl by using synthetic anti-biotic derivatives, IONICS, 2019, 25, 5057–5075.
- N. Arshad, F. Altaf, M. Akram, M. Ullah, Furan and Phenyl Substituted Triazolothiadiazine Derivatives as Copper Corrosion Inhibitors: Electrochemical and DFT Studies, Prot Met Phys Chem Surfaces, 2019, 55, 770–780.
DOI: http://dx.doi.org/10.13171/mjc10502005151417feh
Refbacks
- There are currently no refbacks.
Copyright (c) 2020 Mediterranean Journal of Chemistry
