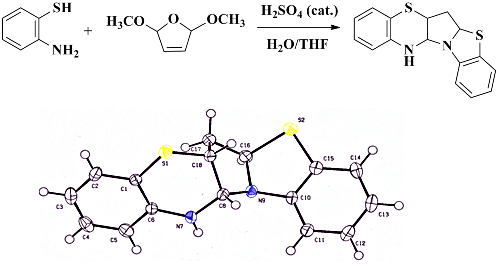
Reaction of 2-aminothiophenol with 2,5-dihydro-2,5-dimethoxyfuran : a facile route to a new dihydrobenzothiazine derivative
Abstract
As part of our studies focused on the design and synthesis of biologically active molecules, polyheterocyclic compound 3 was prepared in one step from commercially available 2-aminothiophenol 1 and 2,5-dihydro-2,5-dimethoxyfuran 2. Derivative 3 underwent a further isomerization to compound 4 in a reaction promoted by traces of HCl in chloroform.
Full Text:
PDFReferences
- a) Y. Sugimoto, T. Tarumi, Q. E. Zhao, Y. Fujii , and C. Kamei, Methods Find Exp. Clin. Pharmacol. 1998, 20, 457; Chem. Abstr. 1999, 130, 75926; b) J. David, and E. J. Wager, Psychopharmacol. 1998, 12, 283; Chem. Abstr. 1999, 130, 119446; c) P. I. Williams, and M. Smith, Eur. J. Anaesthesiol. 1999, 16, 683; Chem. Abstr. 1999, 131, 332076; d) R. Fringuelli, L. Milanese, and F. Schiaffella, Mini Rev. Med. Chem. 2005, 5, 1061-73; e) O. O. Ajani, Arch. Pharm. Chem. Life Sci. 2012, 345, 841-851.
- a) B. S. Rathore, and M. Kumar, Bioorganic & Medicinal Chemistry. 2006, 14, 5678; b) V. V. Dabholkar, and R. P. Gavande, Rasayan J. Chem. 2010, 3, 655; c) Chem. Abstr. 2011, 154, 434836..
- V. Balasubramaniyan, P. Balasubramaniyan, and A. S. Shaikh, Tetrahedron, 1986, 42, 2731.
- M. Avenati, and P. Vogel, Helv. Chim. Acta, 1982, 65, 204.
- CCDC 949457 contains the supplementary crystallographic data for compound 3. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- F. Eiden, and U. Grusdt, Arch. Pharm. (Weinheim, Germany), 1987, 320 (10), 1020.
- V. Facchinetti, R. Reis, R. B. Gomes, R. A. Vasconcelos. Mini Rev. Org. Chem, 2012, 9, 44-53.
DOI: http://dx.doi.org/10.13171/mjc.3.2.2014.20.04.10
Refbacks
- There are currently no refbacks.
Copyright (c) 2015 Mediterranean Journal of Chemistry
