A hitherto unreported impurity in Terazosin – elucidation of the structure, synthesis and cytotoxicity
Abstract
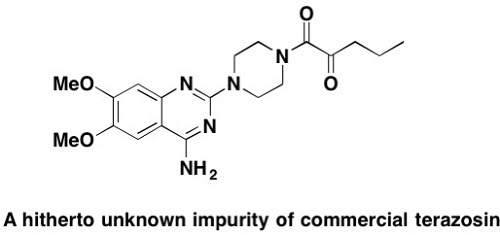
Analysis of several batches of the a1–adrenergic blocking agent terazosin being used as a medication for treating benign prostatic hyperplasia and hypertension revealed the presence of a hitherto not reported impurity. The latter was isolated, and its structure was elucidated from NMR and Mass Spectrometry (MS) data and unambiguously confirmed by independent synthesis. This contamination, represented in 1-[4-(amino-6,7-dimethoxyquinazolin-2-yl)-piperazin-1-yl]-pentane-1,2-dione 2 is likely to occur as the product of a side-reaction in the catalytic hydrogenation step during the synthesis of the drug. Biological screening showed this compound as not cytotoxic for several human tumor cell lines and non-malignant fibroblasts.
Full Text:
PDFReferences
- S.B. Bari, P.S. Jain, A.A. Shirkhedkar, L.V. Sonawane, A.J. Mhaske, J.B. Gawad, Impurities in pharmaceuticals: a review, World J. Pharm. Res., 2015, 4, 2932-2947.
- C.M. Callis, J.P. Bercu, K.M. DeVries, L.K. Dow, D.K. Robbins, D.L. Varie, Risk Assessment of Genotoxic Impurities in Marketed Compounds Administered over a Short-Term Duration: Applications to Oncology Products and Implications for Impurity Control Limits, Org. Process Res. Dev., 2010, 14, 986-992.
- M. Honma, Thresholds of toxicological concern for genotoxic impurities in pharmaceuticals, in: F. Nohmi, S. Fukushima (Eds.) Thresholds of genotoxic carcinogens: From mechanisms to regulation, Academic Press, Amsterdam, 2016, 103-115.
- S. Schmidtsdorff, A.H. Schmidt, Simultaneous detection of nitrosamines and other sartan-related impurities in active pharmaceutical ingredients by supercritical fluid chromatography, J. Pharm. Biomed. Anal., 2019, 174, 151-160.
- M. Batty, R. Pugh, I. Rathinam, J. Simmonds, E. Walker, A. Forbes, S. Anoopkumar-Dukie, C.M. McDermott, B. Spencer, D. Christie, R. Chess-Williams, The role of α1-adrenoceptor antagonists in the treatment of prostate and other cancers, Int. J. Mol. Sci., 2016, 17, 1339.
- M. Oelke, A. Gericke, M.C. Michel, Cardiovascular and ocular safety of α1-adrenoceptor antagonists in the treatment of male lower urinary tract symptoms, Expert Opin. Drug Saf., 2014, 13, 1187-1197.
- A. Wadhawan, A. Banga, Y. Duan, M. Mennesson, Z.H. Wu, Alpha1-Adrenergic Receptor Antagonists Use in Treatment and Prevention of Psychiatric Disorders: A Review, Curr. Psychopharmacol., 2014, 3, 158-183.
- H. Wood, Could a prostate drug be repurposed for Parkinson disease? Nat. Rev. Neurol., 2019, 15, 621.
- J.Q. Yuan, Y. Liu, Z.Y. Yang, X. Qin, K.H. Yang, C. Mao, The efficacy and safety of alpha- 1 blockers for benign prostatic hyperplasia: an overview of 15 systematic reviews, Curr. Med. Res. Opin., 2013, 29, 279-287.
- U. Dunzendorfer, Clinical experience: symptomatic management of BPH with Terazosin, Urology, 1988, 32, 27-31.
- H. Lepor, M. Baumann, E. Shapiro, The alpha-adrenergic binding properties of Terazosin in the human prostate adenoma and canine brain, J. Urol. (Baltimore), 1988, 140, 664-667.
- H. Lepor, D.I. Gup, M. Baumann, E. Shapiro, Laboratory assessment of terazosin and alpha-1 blockade in prostatic hyperplasia, Urology, 1988, 32, 21-26.
- P.M. Parker, The 2019-2024 World Outlook for Pulmonary arterial hypertension (PAH) Therapeutics, Icon Group International, Las Vegas, 2018.
- A.F. Fliri, W.T. Loging, R.A. Volkmann, Drug effects viewed from a signal transduction network perspective, J. Med. Chem., 2009, 52, 8038-8046.
- A. Ghaleiha, K.M. Shahidi, S. Afzali, N. Matinnia, Effect of Terazosin on sweating in patients with major depressive disorder receiving sertraline: A randomized controlled trial, Int. J. Psychiatry Clin. Pract., 2013, 17, 44-47.
- R. Mago, M.E. Thase, B.W. Rovner, Antidepressant-induced excessive sweating: clinical features and treatment with Terazosin, Ann Clin Psychiatry, 2013, 25, 186-192.
- https.//www.parkinson-gesellschaft.de; last accessed 2020-01-22.
- R. Cai, Y. Yuan, Z. Chen, Y. Han, X. Ji, R. Cai, Y. Li, W. Su, L. Gao, X. Ji, L. Liu, Y. Zhang, J.E. Simmering, J.L. Schultz, I. Fernandez-Carasa, A. Consiglio, A. Consiglio, A. Raya, A. Raya, P.M. Polgreen, N.S. Narayanan, C. Zhao, L. Liu, M.J. Welsh, Enhancing glycolysis attenuates Parkinson's disease progression in models and clinical databases, J. Clin. Invest., 2019, 129, 4539-4549.
- J. Xia, B. Feng, Q. Shao, Y. Yuan, W.X. Simon, N. Chen, S. Wu, Virtual screening against phosphoglycerate kinase 1 in quest of novel apoptosis inhibitors, Molecules, 2017, 22, 1029.
- A. Cavalli, F. Lizzi, S. Bongarzone, R. Brun, R. Luise Krauth-Siegel, M.L. Bolognesi, Privileged structure-guided synthesis of quinazoline derivatives as inhibitors of trypanothione reductase, Bioorg. Med. Chem. Lett., 2009, 19, 3031-3035.
- Z. Ma, Y. Lin, Y. Cheng, W. Wu, R. Cai, S. Chen, B. Shi, B. Han, X. Shi, Y. Zhou, L. Du, M. Li, Discovery of the First Environment-Sensitive Near-Infrared (NIR) Fluorogenic Ligand for α1-Adrenergic Receptors Imaging in Vivo, J. Med. Chem., 2016, 59, 2151-2162.
- Z. Ma, Z. Liu, T. Jiang, T. Zhang, H. Zhang, L. Du, M. Li, Discovery of Fluorescence Polarization Probe for the ELISA-Based Antagonist Screening of α1-Adrenergic Receptors, ACS Med. Chem. Lett., 2016, 7, 967-971.
- A. Petty, N. Idippily, V. Bobba, W.J. Geldenhuys, B. Zhong, B. Su, B. Wang, Design and synthesis of small molecule agonists of EphA2 receptor, Eur. J. Med. Chem., 2018, 143, 1261-1276.
- T. Sekiya, H. Hiranuma, S. Hata, S. Mizogami, M. Hanazuka, S. Yamada, Pyrimidine derivatives. 4. Synthesis and antihypertensive activity of 4-amino-2-(4-cinnamoylpiperazino)-6,7-dimethoxyquinazoline derivatives, J. Med. Chem., 1983, 26, 411-416.
- A. Bottini, S.K. De, B. Wu, C. Tang, G. Varani, M. Pellecchia, Targeting Influenza A Virus RNA Promoter, Chem. Biol. Drug Des., 2015, 86, 663-673.
- N.S. Date, V. La Parola, C.V. Rode, M.L. Testa, Ti-doped Pd-Au catalysts for one-pot hydrogenation and ring-opening of furfural, Catalysts, 2018, 8, 252.
- S. Mitsui, Y. Ishikawa, Y. Takeuchi, Hydrogenation end decomposition. 14. Part Catalytic reduction of the furan derivatives with Palladium-und Raney-Nickel-Katalysator, Chem. Zentralbl., 1965, 136, 16538.
- I.F. Bel’skii, N.I. Shuikin, Catalytic hydrogenation and hydrogenolysis of furan compounds, Usp. Khim., 1963, 32, 707-736.
- N.I. Shuikin, R.A. Karakhanov, I. Ibrakhimov, Conversion of tetrahydrofuran homologs over palladized carbon, Izv. Akad. Nauk SSSR Ser. Khim., 1965, 1, 165-167.
- S. Wang, V. Vorotnikov, D.G. Vlachos, A DFT study of furan hydrogenation and ring-opening on Pd(111), Green Chem. 2014, 16, 736-747.
- N.I. Shuikin, I.F. Bel’skii, Hydrogenation of furan compounds over nickel catalysts, Zh. Obshch. Khim., 1959, 29, 3627-3631.
- T. Mizugaki, T. Yamakawa, Y. Nagatsu, Z. Maeno, T. Mitsudome, K. Jitsukawa, K. Kaneda, Direct transformation of furfural to 1,2-pentanediol using a hydrotalcite-supported platinum nanoparticle catalyst, ACS Sustain. Chem. Engin., 2014, 2, 2243-2247.
- M. Kahnt, J. Wiemann, L. Fischer, S. Sommerwerk, R. Csuk, Transformation of asiatic acid into a mitocanic, bimodal-acting rhodamine B conjugate of anomolar cytotoxicity, Eur. J. Med. Chem., 2018, 159, 143-148.
- N. Kyprianou, C.M. Benning, Suppression of human prostate cancer cell growth by a 1-adrenoceptor antagonists doxazosin and Terazosin via induction of apoptosis, Cancer Res., 2000, 16, 4550-4555.
DOI: http://dx.doi.org/10.13171/mjc10502005171436rc
Refbacks
- There are currently no refbacks.
Copyright (c) 2020 Mediterranean Journal of Chemistry
