Electrooxidation of simulated wastewater containing pharmaceutical amoxicillin on thermally prepared IrO2/Ti electrode
Abstract
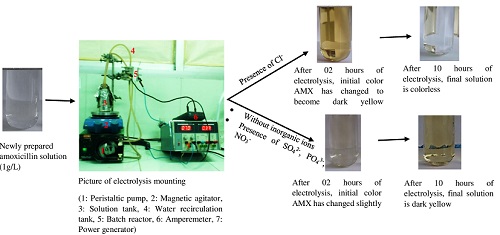
The electrooxidation of amoxicillin (AMX) on the iridium oxide electrode thermally prepared (400°C) has been investigated by cyclic voltammetry and preparative electrolysis. Physical characterization by Scanning Electron Microscopy (SEM) showed that the IrO2 electrode has a rough surface with pores' presence. In cyclic voltammetry, the oxidation of AMX occurs directly at the anode's surface or via the higher degree oxide of iridium oxide (IrO3). It is noted that the oxidation process of AMX can be controlled by diffusion combined with the phenomenon of adsorption. In preparative electrolysis, the effect of several parameters has been investigated. These are the current density, the support medium, the initial pH. The findings obtained show a weak degradation of amoxicillin. The Chemical Oxygen Demand (COD) reduction rate is less than 11% under our experimental conditions, indicating that the IrO2 electrode leads to the parent compound's conversion. Also, the degradation of the organic compound is favored in a very acidic medium.
Furthermore, the effect of inorganic ions such as SO42-, PO43-, NO3-, Cl- was evaluated. Investigations show that these ions' effects are diverse, with COD reduction rates ranging from 2.47%; 2.68%; 7.7%; 16.41%, and 71.65%, respectively, in the absence and the presence of SO42-, PO43-, NO3-, Cl- ions. SO42- have virtually no effect on enhancing the degradation of amoxicillin. PO43- ions provide a slight improvement in amoxicillin degradation. As for nitrate ions, their influence is 2.31 times that of phosphate ions. Chloride ions improve the performance of the electrooxidation of amoxicillin on IrO2 very significantly. The presence of chloride ions makes it possible to go from 2928.35 (absence of inorganic ions) to 33.19 kWh per Kg of COD. This represents an energy gain of over 98%.
Full Text:
PDFReferences
- S. P. Sadia, M. Berté, E. M. H. Loba, F. T. A. Appia, C. Q.-M. Gnamba, S. Ibrahima, L. Ouattara, Assessment of the Physicochemical and Microbiological Parameters of a Teaching Hospital’s Wastewaters in Abidjan in Côte d’Ivoire, J. of Wat. Res. and Protect., 2016, 8, 1251-1265.
- A. Shabnam, Y. Yavuz, S. Zühre, Electrooxidation of tetracycline antibiotic demeclocycline at unmodified boron-doped diamond electrode and its enhancement determination in surfactant-containing media, Talanta, 2021, 223, 121695.
- J. Wang, R. Zhuan, L. Chu, The occurrence, distribution and degradation of antibiotics by ionizing radiation: an overview, Sci. Total Environ., 2019, 646, 1385–1397.
- N. Koch, N. F. Islam, S. Sonowal , R. Prasad, H. Sarma , Environmental antibiotics and resistance genes as emerging contaminants: methods of detection and bioremediation, Curr. Res. Microbial. Sci., 2021. doi:10.1016/j.crmicr.2021.100027.
- J. L. Wilkinson, P. S. Hooda, J. Barker, S. Barton, J. Swinden, Ecotoxic pharmaceuticals, personal care products, and other emerging contaminants: A review of environmental, receptor-mediated, developmental, and epigenetic toxicity with discussion of proposed toxicity to humans, Crit. Rev. Environ. Sci. Technol., 2016, 46, 336 –381.
doi: 10.1080/10643389.2015.1096876.
- J. Du, H. Zhao, Y. Wang, H. Xie, M. Zhu, J. Chen, Presence and environmental risk assessment of selected antibiotics in coastal water adjacent to mariculture areas in the Bohai Sea, Ecotoxicol. and Environ. Saf., 2019, 177, 117-123.
- A. Ezzariai, M. Hafidi, A. Khadra, Q. Aemig, L. El Fels, M. Barret, G. Merlina, D. Patureau, E. Pinelli, Hum- an and veterinary antibiotics during composting of sludge or manure: global perspectives on persistence, deg- radation, and resistance genes, J. Hazard Mater., 2018, 359, 465–481. doi: 10.1016/j.jhazmat.2018.07.092.
- L. Patrolecco, J. Rauseo, N. Ademollo, P. Grenni, M. Cardoni, C. Levantesi, M. L. Luprano, A. B. Caraccio- lo, Persistence of the antibiotic sulfamethoxazole in river water alone or in the co-presence of ciprofloxacin, Sci. Tot. Environ., 2018, 640-64, 1438-1446.
- H. Q. Anh, T. P. Q. Le, N. D. Le, X. X. Lu, T. T. Duong, J. Garnier, E. Rochelle-Newall, S. Zhang, O. Neung- Hwan, C. Oeurng, C. Ekkawatpanit, T. D. Nguyen, Q. T. Nguyen, T. D. Nguyen, T. N. Nguyen, T. L. Tran, T. Kunisue, R. Tanoue, S. Takahashi, T.B. Minh, H.T. Le, T.N.M. Pham, T.A.H. Nguyen, Antibiotics in surface water of East and Southeast Asian countries: A focused review on contamination status, pollution sources, potential risks, and future perspectives, Sci. of the Tot. Environ., 2020.
doi: 10.1016/j.scitotenv.2020.142865.
- M. Gavrilescu, K. Demnerová, J. Aamand, S. Agathos, F. Fava, Emerging pollutants in the environment: present and future challenges in biomonitoring, ecological risks and bioremediation, N. Biotech., 2015, 32 (1), 147–156.
- S. Bergeron, B. Raj, N. Rajkumar, A. Corbin, G. LaFleur, Presence of antibiotic resistance genes in raw source water of a drinking water treatment plant in a rural community of USA, Int. Biodeterior. Biodegrad., 2017, 1-7.
- P. Kovalakova, L. Cizmas, T. J. McDonald, B. Marsalek, M. Feng, V. K. Sharma, Occurrence and toxicity of antibiotics in the aquatic environment: A review, Chemosphere, 2020, 251, 126351.
- M. Klavarioti, D. Mantzavinos, D. Kassinos, Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes, Environ. Int., 2009, 35, 402-417.
- B. Yang, G. Chen, G. Chen, Submerged membrane bioreactor in treatment of simulated restaurant wastewater, Sep. Purif. Technol., 2012, 88, 184-190.
- A. Radka, T. Kümpel, K. Kümmerer, Assessment of degradation of 18 antibiotics in the Closed Bottle Test, Chemosphere, 2004, 57(6), 505–512.
- M. Klavarioti, D. Mantzavinos, D. Kassinos, Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes, Environ. Int, 2009, 35, 402–417.
- M. F. García-Montoya, S. Gutiérrez-Granados, A. Alatorre-Ordaz, R. Galindo, R. Ornelas, J. M. Peralta- Hernández, Application of electrochemical/BDD process for the treatment wastewater effluents containing pharmaceutical compounds, J. Ind. Eng. Chem, 2015, 31,
-243.
- L. P. Bao, C. O. Chong, S. M. S. Mohamed, S. Pau-Loke, C. Jo-Shu, C. L. Tau, S. L. Su, C. J. Joon, Conventional and Emerging Technologies for Removal of Antibiotics from Wastewater, J. Hazar. Mater., 2020.
doi: 10.1016/j.jhazmat.2020.122961.
- V. Punturat, K.-L. Huang, Degradation pathways and organic matter transformation of acesulfame potassium electrooxidation in real water matrices, J. Taiwan Inst. Chem. Eng., 2017, 80, 222-230.
- J. Mora-Gomez, E. Ortega, S. Mestre, V. Pérez-Herranz, M. García-Gabaldón, Electrochemical degradation of norfloxacin using BDD and new Sb-doped SnO2 ceramic anodes in an electrochemical reactor in the presence and absence of a cation-exchange membrane, Sep. Pur. Technol., 2018. doi: 10.1016/j.seppur.2018.05.017.
- J. L. D. S. Duarte, A. M. S. Solano, M. L. P. M. Arguelho, J. Tonholo, C. A. Martínez-Huitle, C. L. de Paiva, S. Zant, Evaluation of treatment of effluents contaminated with rifampicin by Fenton, electrochemical and associated processes, J. Water Process Eng., 2018, 22, 250-257.
- E. Brillas, C. A. Martínez-Huitle, Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review, Appl. Catal. B Environ., 2015, 166-167, 603-643.
- S. Vasilie, F. Manea, A. Baciu, A. Pop, Dual use of boron-doped diamond electrode in antibiotics-containing water treatment and process control, Process Saf. Environ., 2018, 117, 446-453.
- Z. Frontistis, M. Antonopoulou, D. Venieri, I. Konstantinou, D. Mantzavinos, Boron-doped diamond oxidation of amoxicillin pharmaceutical formulation: Statistical evaluation of operating parameters, reaction pathways and antibacterial activity, J. Environ. Manage., 2017, 195 (2), 100-109.
- Q. Dai, J. Zhou, M. Weng, X. Luo, D. Feng, J. Chen, Electrochemical oxidation metronidazole with Co modified PbO2 electrode: Degradation and mechanism, Sep. Purif. Technol., 2016, 166, 109-116.
- D. Rosestolato, J. Fregoni, S. Ferro, A. De Battisti, Influence of the nature of the electrode material and process variables on the kinetics of the chlorine evolution reaction. The case of IrO2-based electrocatalysts, Electrochim. Acta, 2014, 139, 180–189.
doi: 10.1016/j.electacta.2014.07.037.
- D. Sun, X. Hong, K. Wu, K. S. Hui, Y. Du, K. N. Hui, Simultaneous removal of ammonia and phosphate by electrooxidation and electrocoagulation using RuO2-IrO2/Ti and microscale zero-valent iron composite electrode, water Res., 2020, 169, 115239.
- F. Zaviska, P. Drogui, J.-F. Blais, G. Mercier,P. Lafrance, Experimental design methodology applied to electrochemical oxidation of the herbicide atrazine using Ti/IrO2 and Ti/SnO2 circular anode electrodes, J. Hazard. Mater., 2011, 185, 1499-1507.
- M. Li, C. Feng, W. Hub, Z. Zhanga, N. Sugiura, Electrochemical degradation of phenol using electrodes of Ti/RuO2–Pt and Ti/IrO2–Pt,
J. Hazard. Mater., 2009, 162, 455-462.
- K. Ollo, P. L. A. Guillaume, A. F. T. Auguste, G. C. Quand-Meme, K. K. Honoré, O. Lassiné, Influence of various metallic oxides on the kinetic of the oxygen evolution reaction on platinum electrodes, J. Electrochem. Sci. Eng., 2015, 5(2), 79-91. doi: 10.5599/jese.157.
- L. Bao, W. Chengyan, C. Yongqiang, M. Baozhong, Electrochemical behavior and corrosion mechanism of Ti/IrO2-RuO2 anodes in sulphuric acid solution, J. Electroanal. Chem., 2019, 837, 175-183.
- H. Cao, M. Chen, L. Wu, G. Hou, Y. Yiping Tang, G. Zheng, Electrochemical properties of IrO2 active anode with TNTs interlayer for oxygen evolution, Appl. Surf. Sci., 2018, 428, 861–869.
- S. D. Jojoa-Sierra, J. Silva-Agredo, E. Herrera-Calderon, R. A. Torres-Palma, Elimination of the antibiotic norfloxacin in municipal wastewater, urine and seawater by electrochemical oxidation on IrO2 anodes, Sci. Total Environ., 2017, 575, 1228-1238.
- A. L. Giraldo, E. D. Erazo-Erazo, O. A. Florez-Acosta, E. A. Serna-Galvis, R. A. Torres Palma, Degradation of the antibiotic oxacillin in water by anodic oxidation with Ti/IrO2 anodes: Evaluation of degradation routes, organic by-products and effects of water matrix components, Chem. Eng. J., 2015, 279, 103–114.
- E. Chatzisymeon, A. Dimou, D. Mantzavinos, A. Katsaounis, Electrochemical oxidation of model compounds and olive mill wastewater over DSA electrodes: 1. The case of Ti/IrO2 anode, J. Hazard. Mater., 2009, 167, 268–274.
- E. Chatzisymeon, S. Fierro, I. Karafyllis, D. Mantzavinos, N. Kalogerakis, A. Katsaounis, Anodic oxidation of phenol on Ti/IrO2 electrode: Experimental studies, Catal. Today, 2010, 151, 185–189.
- N. Jiang, Y. Wang, Q. Zhao, Z. Ye, Application of Ti/IrO2 electrode in the electrochemical oxidation of the TNT red water, Environ. Pollut., 2020, doi: 10.1016/j.envpol.2019.113801.
- F. Sopaj, M. A. Rodrigo, N. Oturan, F. I. Podvorica, J. Pinson, M. A. Oturan, Influence of the anode materials on the electrochemical oxidation efficiency. Application to oxidative degradation of the pharmaceutical amoxicillin, Chem. Eng. J., 2015, 262, 286-294.
- A. M. S. Sales, C. K. C. Araujo, J. V. Melo, J. M. Peralta-Hernandez, D. R. Silva, C. A. Martinez-Huitle, Decontamination of real textile industrial effluent by strong oxidant species electrogenerated on diamond electrode: viability and disadvantages of this electrochemical technology, Appl. Catal. B, 2013, 130–131,
–120.
- M. G. Tavares, L.V. A. Silva, A. M. S. Sales, J. Tonholo, C. A. Martinez-Huitle, C. L. P. S. Zanta, Electrochemical oxidation of Methyl Red using Ti/Ru0,3Ti0,7 O2 anodes, Chem. Eng. J., 2012, 204–206, 141–150.
- O. I. Anglada, A. Urtiaga, I. Ortiz, Contributions of electrochemical oxidation to wastewater treatment: fundamentals and review of applications, J. Chem. Technol. Biotechnol., 2009, 84, 1747–1755.
- S. Periyasamy, M. Muthuchamy, Electrochemical oxidation of paracetamol in water by graphite anode: Effect of pH, electrolyte concentration and current density. J. Environ. Chem. Eng., 2018.
doi: 10.1016/j.jece.2018.08.036.
- S. A. Neto, A. R. de Andrade, Electrooxidation of glyphosate herbicide at different DSAR compositions: pH, concentration and supporting electrolyte effect, Electrochim. Acta, 2009, 54, 2039-2045.
- C. Q. M Gnamba, F. T. A. Appia, E. M. H. Loba, M. Berthe, S. P. Sadia, S. Ibrahima, L. Ouattara, Electrooxidation of Ceftriaxone in Its Commercial Formulation on Boron Doped Diamond Anode, J. Adv. Electro- chem., 2016, 2(2), 85–88.
- R. E. Palma-Goyes, J. Silva-Agredo, J. Vazquez-Arenas, I. Romero-Ibarra, R. A. Torres-Palma, The effect of different operational parameters on the electrooxidation of Indigo Carmine on Ti/IrO2-SnO2-Sb2O3, J. Environ. Chem. Eng., 2018. doi: 10.1016/j.jece.2018.04.035.
- N. Wachter, J. M. Aquino, M. Denadai, J. C. Barreiro, A. Jose Silva, Q. B. Cass, N. Bocchi, R. C. Rocha- Filho, Electrochemical degradation of the antibiotic ciprofloxacin in a flow reactor using distinct BDD anodes: Reaction kinetics, identification and toxicity of the degradation products, Chemosphere, 2019, 234, 461- 470.
- L. A. Perea, R. E. Palma-Goyes, J. Vazquez-Arenas, I. Romero-Ibarra, C. Ostos, R. A. Torres-Palma, Efficient cephalexin degradation using active chlorine produced on ruthenium and iridium oxide anodes: Role of bath composition, analysis of degradation pathways and degradation extent, Sci. Total Environ., 2019, 648, 377– 387.
- S. Hong, T-k. Lee, M. R. Hoffmann, K. Cho, Enhanced chlorine evolution from dimensionally stable anode by heterojunction with Ti and Bi-based mixed metal oxide layers prepared from nanoparticle slurry, J. Catal., 2020.
doi: 10.1016/j.jcat.2020.04.009.
- K. Cho, M. R. Hoffmann, BixTi1-xOz functionalized heterojunction anode with an enhanced reactive chlorine generation efficiency in diluted aqueous solutions, Chem. Mater., 2015, 27, 2224–2233.
- F. Bonfatti, S. Ferro, F. Lavezzo, M. Malacarne, G. Lodi, A. De Battisti, 2000. Electrochemical incineration of glucose as a model organic substrate. II role of active chlorine mediation, J. Electrochem. Soc., 2000, 147, 592–596.
- P. Cañizares, F. Larrondo, J. Lobato, M. A. Rodrigo, C. Sáez, Electrochemical synthesis of peroxodiphosphate using boron-doped anodes, J. Electrochem. Soc., 2005, pD191-D196.
- P. Cañizares, C. Sáez, J. Lobato, M. A. Rodrigo, Electrochemical Oxidation of Polyhydroxybenzenes on Boron-Doped Diamond Anodes, Ind. Eng. Chem. Res., 2004, 43, 6629-6637.
- K. P. De Amorim, L. L. Romualdo, L. S. Andrade, Electrochemical degradation of sulfamethoxazole and trimethoprim at boron-doped diamond electrode: Performance, kinetics and reaction pathway, Sep. Purif. Technol., 2013, 120, 319–327.
DOI: http://dx.doi.org/10.13171/mjc02104071566ftaa
Refbacks
- There are currently no refbacks.
Copyright (c) 2021 Mediterranean Journal of Chemistry
