Synthesis of new bimetallic phosphate (Al/Ag3PO4) and study for its Catalytic performance in the synthesis of 1,2-dihydro-l-phenyl-3H-naphth [1,2-e]-[1,3] oxazin-3-one derivatives
Abstract
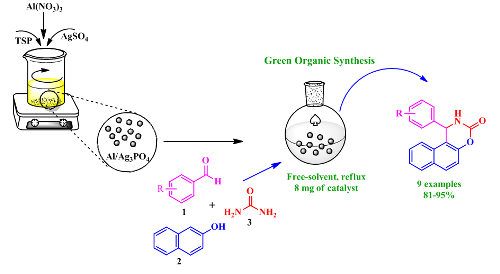
This work aims to prepare a new bimetallic phosphate catalyst using a new simple and effective method. This new catalyst was ready for the first time by a modification of Triple Super Phosphate (TSP) fertilizer with silver sulfate (AgSO4), followed by the impregnation of the aluminum atoms using aluminum nitrate (Al(NO3)3). The use of Al/Ag3PO4, for the first time as a heterogeneous catalyst in organic chemistry, offers a new, efficient, and green pathway for synthesizing 1,2-dihydro-l-phenyl-3H-naphth[1,2-e]-[1,3]oxazin-3-one derivatives by one-pot three-component cyclocondensation of b-naphthol, aryl aldehyde, and urea. The structure and the morphology of the prepared catalyst were characterized by spectroscopic methods such as X-Ray Diffraction (XRD), Fourier Transform Infrared spectroscopy (FT-IR), and dispersive X-ray spectrometry coupled with a scanning electron microscope (EDX-SEM). In addition, the optimization of the reaction parameters was carried out considering the effect of catalyst amount, the temperature, and the solvent. The procedure described herein allowed a comfortable preparation of oxazine derivatives with excellent yields, short reaction times, and in the absence of organic solvent.
Full Text:
PDFReferences
N.S. Alavijeh, B.A. Arndtsen, S. Balalaie, D. Bonne, C. Chen, Multicomponent Reactions: Reactions Involving an α,ß-unsaturated carbonyl compound as electrophilic component, Cycloadditions and Boron-, Silicon-, Free-Radical- and Metal-Mediated Reactions, Thieme Verlag, Stuttgart, New York, 2014, 2.
C.C.A. Cariou, G.J. Clarkson, M. Shipman, Rapid synthesis of 1,3,4,4-tetrasubstituted β-lactams from methylene aziridines using a four-component reaction, J. Org. Chem., 2008, 73, 9762–9764.
A. Kumar, M.K. Gupta, M. Kumar, Micelle promoted supramolecular carbohydrate scaffold-catalyzed multicomponent synthesis of 1,2-dihydro-1-aryl-3H-naphth[1,2-e][1,3]oxazin-3-one and amidoalkyl naphthols derivatives in aqueous medium, RSC Adv., 2012, 2, 7371.
M.N. Chen, L.P. Mo, Z.S. Cui, Z.H. Zhang, Magnetic nanocatalysts: Synthesis and application in multicomponent reactions, Curr. Opin. Green Sustain., 2019, 15, 27–37.
C.G. Neochoritis, T. Zhao, A. Dömling, Tetrazoles via multicomponent reactions, Chem. Rev., 2019, 119, 1970–2042.
M.A.A. Radwan, M.A. Shehab, S.M. El-Shenawy, Synthesis and biological evaluation of 5-substituted benzo[b]thiophene derivatives as anti-inflammatory agents, Monatsh. Chem., 2009, 140, 445–450.
M. Dabiri, A.A. Mohammadi, H. Qaraat., An efficient and convenient protocol for the synthesis of novel 1′H-spiro[isoindoline-1,2′-quinazoline]-3,4′(3′H)-dione derivatives, Monatsh. Chem., 2009, 140, 401–404.
R.W. Armstrong, A.P. Combs, P.A. Tempest, S.D. Brown, T.A. Keating, Multiple-component condensation strategies for combinatorial library synthesis, Acc. Chem. Res., 1996, 29, 123–131.
M. Zhang, Y.H. Liu, Z.R. Shang, H.C. Hu, Z.H. Zhang, Supported molybdenum on graphene oxide/Fe3O4: An efficient, magnetically separable catalyst for one-pot construction of spiro-oxindole dihydropyridines in deep eutectic solvent under microwave irradiation, Catalysis Communications, 2017, 88, 39–44.
P. Kaur, B. Kumar, V. Kumar, R. Kumar, Chitosan-supported copper as an efficient and recyclable heterogeneous catalyst for A3/decarboxylative A3-coupling reaction, Tetrahedron Lett., 2018, 59, 1986–1991.
Y.S. Wagh, D.N. Sawant, K.P. Dhake, K.M. Deshmukh, B.M. Bhanage, Allylation of 1-phenyl-1-propyne with N- and O-pronucleophiles using polymer-supported triphenylphosphine palladium complex as a heterogeneous and recyclable catalyst, Tetrahedron Lett., 2011, 52, 5676–5679.
S. Chandrasekhar, V. Patro, L.N. Chavan, R. Chegondi, R. Grée, Multicomponent reactions in PEG-400: ruthenium-catalyzed synthesis of substituted pyrroles, Tetrahedron Lett., 2014, 55, 5932–5935.
P. Slobbe, E. Ruijter, R.V.A. Orru, Recent applications of multicomponent reactions in medicinal chemistry, Med. Chem. Commun., 2012, 3, 1189.
A. Dömling, W. Wang, K. Wang, Chemistry and biology of multicomponent reactions, Chem. Rev., 2012, 112, 3083–3135.
M. Patel, S.S. Ko, R.J. McHugh, Synthesis and evaluation of analogs of efavirenz (sustivatm) as hiv-1 reverse transcriptase inhibitors, Bioorg. Med. Chem. Lett., 1999, 9, 2805–2810.
L.D.S. Yadav, R. Kapoor, Novel salicylaldehyde-based mineral-supported expeditious synthesis of benzoxazin-2-ones, J. Org. Chem., 2004, 69, 8118–8120.
I. Szatmári, A. Hetényi, L. Lázár, F. Fülöp, Transformation reactions of the betti base analog aminonaphthols, J. Heterocycl. Chem., 2004, 41, 367–373.
M.A. Zolfigol, M. Safaiee, F. Afsharnadery, Silica vanadic acid [SiO2–VO(OH)2] as an efficient heterogeneous catalyst for the synthesis
of 1,2-dihydro-1-aryl-3H-naphth[1,2-e][1,3]oxazin-3-one and 2,4,6-triarylpyridine derivatives via anomeric based oxidation, RSC Adv., 2015, 5, 100546–100559.
A. Chaskar, V. Vyavhare, V. Padalkar, H. Deokar, An environmentally benign one-pot synthesis of 1,2-dihydro-1-aryl-3H- naphth[1,2-e] [1,3]oxazin-3-one derivatives catalyzed by phosphomolybdic acid, Journal of the Serbian Chemical Society, 2011, 1, 21-26.
M. Dabiri, A. Delbari, A. Bazgir, A Novel Three-Component, One-Pot synthesis of 1,2-dihydro-1-arylnaphtho[1,2- e ][1,3]oxazine-3-one derivatives under microwave-assisted and thermal solvent-free conditions, Synlett, 2007, 2007, 0821–0823.
P. Rahmani, F.K. Behbahani, A one-pot synthesis of 1,2-dihydro-1-arylnaphtho[1,2-e][1,3]oxazine-3-ones catalyzed by iron(III) phosphate under solvent-free condition, Inorg. Nano-Met. Chem., 2017, 47, 713-716.
K.N. Reddy, S. Ramanaiah, N.A.K. Reddy, Chitosan Catalyzed One-Pot Three-Component Conventional Synthesis of 1, 2-Dihydro-1-Arylnaphtho [1, 2-e][1, 3] Oxazine-3-Ones, IJRR., 2019, 6, 85-93.
F. Nemati, A. Beyzai, A facile one-pot solvent-free synthesis of 1,2-dihydro-1-arylnaphtho[1,2-e][1,3]oxazine-3-ones catalyzed by wet cyanuric chloride, J. Chem., 2013, 2013, 1–4.
S. Gajare, A. Patil, D. Kale, Graphene oxide-supported ionic liquid phase catalyzed synthesis of 3,4-dihydro-2h-naphtho[2,3-e][1,3]oxazine-5,10-diones, Catal Lett., 2020, 150, 243–255.
G. Harichandran, P. Parameswari, P. Shanmugam, A one-pot multicomponent synthesis of naphthoxazin-3-one derivatives using amberlite IRA-400 Cl resin as green catalyst, LOC, 2018, 15, 600–605.
G. Dou, F. Sun, X. Zhao, D. Shi, An efficient synthesis of 3,3′,4,4′-tetrahydro-4,4′-bibenzo[e][1,3]oxazine-2,2′-dione derivatives with the aid of low-valent titanium, Chin. J. Chem., 2011, 29, 2465–2470.
A.E. Hallaoui, S. Chehab, T. Ghailane, B. Malek, O. Zimou, S. Boukhriss, A. Souizi, R. Ghailane, Application of Phosphate Fertilizer Modified by Zinc as a Reusable Efficient Heterogeneous Catalyst for the Synthesis of Biscoumarins and Dihydropyrano[3,2-c]Chromene-3-Carbonitriles under Green Conditions, Polycyclic Aromatic Compounds, 2020, 1–20.
A. El Hallaoui, S. Chehab, B. Malek, O. Zimou, T. Ghailane, S. Boukhriss, A. Souizi, R. Ghailane, Valorization of the Modified Mono Ammonium Phosphate by Cobalt in the Synthesise of 3,4‐Dihydropyrano[c]chromene Derivatives, ChemistrySelect, 2019, 4 (11), 3062–3070.
S. Chehab, Y. Merroun, T. Ghailane, N. Habbadi, S. Boukhriss, A. Hassikou, R. Ghailane, M. Akhazzane, A. Kerbal, A. Daich, A. Souizi, A new process for Na2Ca(HPO4)2 synthesis and its application as a heterogeneous catalyst in Knoevenagel condensation, Mediterr.J.Chem., 2018, 7, 56–67.
Y. Merroun, S. Chehab, T. Ghailane, M. Akhazzane, A. Souizi, R. Ghailane, Preparation of tin-modified mono-ammonium phosphate fertilizer and its application as heterogeneous catalyst in the benzimidazoles and benzothiazoles synthesis, Reac Kinet Mech Cat., 2019, 126, 249–264.
R. Qu, W. Zhang, N. Liu, Antioil Ag3PO4 Nanoparticle/Polydopamine/Al2O3 Sandwich Structure for Complex Wastewater Treatment: Dynamic Catalysis under Natural Light, ACS Sustainable Chem. Eng., 2018, 6, 8019–8028.
N. Saheb, M. Shahzeb Khan, A.S. Hakeem, Effect of processing on mechanically alloyed and spark plasma sintered Al-Al2O3 Nanocomposites, J. Nanomater, 2015, 2015, 1–13.
A. Monshi, M.R. Foroughi, M. Monshi, Modified Scherrer Equation to Estimate More Accurately Nano-Crystallite Size Using XRD, World Journal of Nano Science and Engineering, 2012, 2, 154–160.
M. Wang, Y. Liang, T.T. Zhang, J.J. Gao, Three-component synthesis of amidoalkyl naphthols catalyzed by bismuth(III) nitrate pentahydrate, Chin. Chem. Lett., 2012, 23, 65–68.
F. Dong, Y. Li-fang, Y. Jin-ming, Synthesis of 1,2-dihydro-1-arylnaphtho[1,2-e][1,3]oxazine-3-one catalyzed by pyridinium-based ionic liquid, Res Chem Intermed., 2013, 39 (6), 2505–2512.
Y. Wang, J. Zhou, K. Liu, L. Dai, Bi-SO3H-functionalized room temperature ionic liquids based on bipyridinium: highly efficient and recyclable catalysts for the synthesis of naphthalene-condensed oxazinone derivatives, RSC Adv., 2013, 3, 9965.
C.V. Subbareddy, S. Sundarrajan, A. Mohanapriya, R. Subashini, S. Shanmugam, Synthesis, antioxidant, antibacterial, solvatochromism and molecular docking studies of indolyl-4H-chromene-phenylprop-2-en-1-one derivatives, J. Mol. Liq., 2018, 251, 296–307.
A. Kumar, A. Saxena, M. Dewan, A. De, S. Mozumdar, Recyclable nanoparticulate copper-mediated synthesis of naphthoxazinones in PEG-400: a green approach, Tetrahedron Lett., 2011, 52 (38), 4835–4839.
R. Hunnur, R. Kamble, A. Dorababu, B.S. Kumar, C. Bathula, TiCl4: An efficient catalyst for one-pot synthesis of 1, 2-dihydro-1-aryl-naphtho-[1, 2-e][1, 3] oxazin-3-one derivatives and their drug score analysis, Arab. J. Chem., 2017,10, S1760-S1764.
A. Chaskar, V. Vyavhare, V. Padalkar, K. Phatangare, H. Deokar, An environmentally benign one-pot synthesis of 1,2-dihydro-1-arylnaphtho[1,2-e][1,3]oxazine-3-one derivatives catalysed by phosphomolybdic acid, J. Serb. Chem., 2011, 76, 21–26.
F. Sheikholeslami-Farahani, The Green Procedure for the synthesis of 1, 3-oxazine-4- thiones Using ZnO Nanoparticles, J. Appl. Chem., 2015, 6.
H. Abbastabar Ahangar, G.H. Mahdavinia, K. Marjani, A. Hafezian. One-pot synthesis of 1,2-dihydro-1-arylnaphtho[1,2-e][1,3]oxazine-3-one derivatives catalyzed by perchloric acid supported on silica (HClO4/SiO2) in the absence of solvent, J. Iran. Chem. Soc., 2010, 7, 770–774.
R. Nongrum, M. Kharkongor, G.S. Nongthombam, [1,3]oxazines: green synthesis by sonication using a magnetically-separable basic nano-catalyst and investigation of its activity against the toxic effect of a pesticide on the morphology of blood cells, Environ Chem Lett., 2019, 17, 1325–1331.
S. Azad, B.B.F. Mirjalili, One-pot solvent-free synthesis of 2,3-dihydro-2-substituted-1H-naphtho[1,2-e][1,3]oxazine derivatives using Fe3O4@nano-cellulose/TiCl as a bio-based and recyclable magnetic nano-catalyst, Mol Divers, 2019, 23, 413–420.
E. Babaei, B.B.F. Mirjalili, One-pot aqueous media synthesis of 1,3-oxazine derivatives catalyzed by reusable nano-Al2O3/BF3/Fe3O4 at room temperature, Polycyclic Aromatic Compounds, 2019, 1–8.
A.H. Kategaonkar, S.S. Sonar, K.F. Shelke, B.B. Shingate, M.S. Shingare, ionic liquid catalyzed multicomponent synthesis of 3, 4-dihydro-3-substituted-2H-naphtho [2,1-e][1, 3] oxazine derivatives, Organic Communications, 2009, 3, 1-7.
DOI: http://dx.doi.org/10.13171/mjc02108091579elhallaoui
Refbacks
- There are currently no refbacks.
Copyright (c) 2021 Mediterranean Journal of Chemistry
