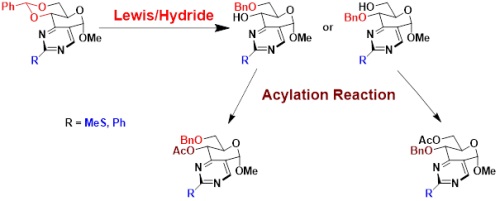
Regioselective reduction of benzylidene acetals from a bis-heterocyclic pyrimidino-pyranoside platform
Abstract
After the preparation with few steps of the original bicyclic osidic scaffold of pyrimidino-pyranoside type, the exploration of the reactivity of the pyranose part allowed us to carry out different procedures for opening 4,6-O-benzylidene.
Full Text:
PDFReferences
R. Hirschmann, K.C. Nicolaou, S. Pietranico, J. Salvino, E.M. Leahy, P.A. Sprengeler, G. Furst, A.B. Smith III, Nonpeptidal peptidomimetics with .beta.-D-glucose scaffolding. A partial somatostatin agonist bearing a close structural relationship to a potent, selective substance P antagonist, J. Am. Chem. Soc., 1992, 114, 9217-9218.
N. Moitessier, S. Dufour, F. Chrétien, J.P. Thiery, B. Maigret, Y. Chapleur, Design, synthesis and preliminary biological evaluation of a focused combinatorial library of stereodiverse carbohydrate-scaffold-based peptidomimetics, Bioorg. Med. Chem., 2001, 9, 511–523.
N. Moitessier, C. Henry, B. Maigret, Y. Chapleur, Combining pharmacophore search, automated docking, and molecular dynamics simulations as a novel strategy for flexible docking. Proof of concept: docking of arginine− glycine− aspartic acid-like compounds into the αvβ3 binding site, J. Med. Chem., 2004, 47, 4178-4187.
C. Henry, N. Moitessier, Y. Chapleur, Vitronectin receptor alpha (V) beta (3) integrin antagonists: chemical and structural requirements for activity and selectivity, Mini Rev. Med. Chem., 2002, 2, 531-542.
S. K. Bozhena,V.M. Orekhova, E.T. Yury, N.E. Nifantiev, Is an acyl group at O-3 in glucosyl donors able to control α-stereoselectivity of glycosylation? The role of conformational mobility and the protecting group at O-6, Carbohydrate Research, 2014, 384, 70-86.
Erika Mező, Mihály Herczeg, Fruzsina Demeter, Ilona Bereczki, Magdolna Csávás, Anikó Borbás. J. Org. Chem. 2021, 86, 18, 12973–12987.
T. Madhubabu, S.K. Yousuf, S. Aravinda, B. Singh, D. Mukherjee, Cyanuric chloride/sodium borohydride: a new reagent
combination for reductive opening of 4, 6-benzylidene acetals of carbohydrates to primary alcohol, Carbohydrate Research, 2013, 381,
-145.
A. Mitra, B. Mukhopadhyay, Linear synthesis of the hexasaccharide related to the repeating unit of the O-antigen from Shigella flexneri serotype 1d (I: 7, 8), Carbohydrate Research, 2016, 426, 1-8.
A. Aravind, S. Baskaran, 1, 3: 4, 6-Di-O-benzylidene-d-mannitol as a source for novel chiral intermediates through regioselective reductive cleavage, Tetrahedron Letters, 2005, 46, 743-745.
S. Ed. Hanessian, Marcel Dekker, Inc.: New York, 1997, 53–67.
T.V. Wang, A.V. Demchenko, Synthesis of carbohydrate building blocks via regioselective uniform protection/deprotection strategies, Org. Biomol. Chem., 2019, 17, 4934–4950.
J. Janssens, M.D.P. Risseeuw, J. Van der Eycken, S. Van Calen-bergh, Regioselective ring opening of 1, 3-dioxane-type acetals in carbohydrates, Eur. J. Org. Chem., 2018, 46, 6405–6431.
Victoria Dimakos and Mark S. Taylor. Chem. Rev. 2018, 118, 11457−11517.
I. Samb, N.P. Moïse, S.L. langle, Y. Chapleur, Efficient functionalizations of a pyranosido-pyrimidine scaffold, Tetrahedron, 2009, 65,896-902.
DOI: http://dx.doi.org/10.13171/mjc02109271584samb
Refbacks
- There are currently no refbacks.
Copyright (c) 2021 Mediterranean Journal of Chemistry
