Design of new dihydrothiophenone derivatives with improved anti-malaria activity
Abstract
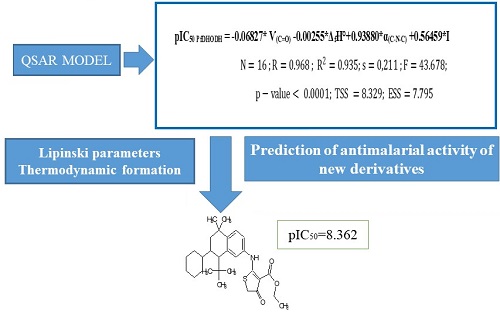
This study uses quantum chemical methods to design new dihydrothiophenone derivatives with improved antimalarial activity. The molecules were optimized using the B3LYP/6-31G (d, p) level of theory. The application of the MLR method of the XLSTAT program allowed the development of a regression model. The developed model's statistical indicators (R²=93.50 %, S=0.211, F=43.678) attest to the robustness and reliability. After the study on the substituents influencing the antimalarial activity, thirty (30) new CDH-coded molecules were generated, considering these effects. Twenty-four (24) of these new molecules showed higher values of inhibitory concentration potential than the parent compound (pIC50= 7.036). In addition, the thermodynamic formation quantities formed at 298K were calculated. Lipinski's rule and antimalarial activities proved that these twenty-four new molecules could be used as antimalarial drugs.
Full Text:
PDFReferences
M. J. Crutcher et SL. Hoffman, Medical Microbiology, 4th éd. University of Texas Medical Branch at Galveston: Baron S, 1996. [online]. Available on: https://www.ncbi.nlm.nih.gov/books/NBK8584/
N. C. Wells, R. H. Van Huijsduijnen, W. C. Van Voorhis, Malaria medicines: A glass half full?, Nat. Rev. Drug Discov., 2015, 15, 424‑442.
N. Burrows, E. Burlot, B. Campo, S. Cherbuin, S. Jeanneret, D. Leroy, Antimalarial drug discovery the path towards eradication, Parasitology, 2014, 141, 128‑139.
F. Konaté, F. Diarrassouba, G. S. Dembélé,
M. G. R. Koné, B. Konaté, N. Ziao, Elaboration of a predictive QSAR Model of the Antipaludial Activity of a Series of Dihydrothiophénone Molecules at theory level B3LYP/6-31G(d,p), Chemical Science International Journal, 2021, 30, 1‑12.
L. Kuo, Fragment-based drug design : tools, practical approaches and examples. Methods in enzymology, Academic Press, 2011, 493.
M. J. Frisch et al., Gaussian 09, Wallingford CT: Gaussian, Inc, 2009.
M. W. Chase, C. A. Davies, J. R. Downey, D. J. Frurip, R. A. McDonald, A. N. Syverud, JANAF Thermochemical Tables, J. Phys. Ref., 1985, 14.
C. Lipinski, F. Lombardo, B. Dominy, P. J. Feeney, Experimental and Computational approaches to estimate solubility and permeability in drug discovery and development settings, Adv Drug Deliv Rev, 1997, 46, 3‑26.
M. Xu, Novel selective and potent inhibitors of malaria parasite dihydroorotate dehydrogenase: Discovery and optimization of dihydrothiophenone derivatives, J. Med. Chem., 2013, 56, 7911‑7924.
DOI: http://dx.doi.org/10.13171/mjc02112181610dembele
Refbacks
- There are currently no refbacks.
Copyright (c) 2021 Mediterranean Journal of Chemistry
