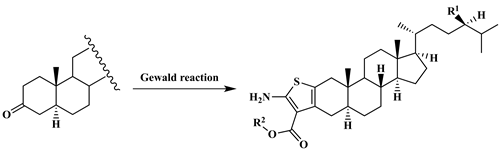
Synthesis and characterization of steroidal, anellated aminothiophenes by Gewald reaction
Abstract
Gewald 3-component reactions (G-3CR), i.e., reactions of a carbonyl compound with an activated nitrile in the presence of a secondary amine and sulfur, lead straightforwardly to anellated 2-aminothiophenes. Interestingly, their application to steroidal hydrocarbons has been limited to a single example. We could show in this work that Gewald 3-component reactions can be performed successfully for molecules holding a cholesterol or sitostanol skeleton, such as 5a-cholestan3-one (8) and 5a-sitostan-3-one (11), thus leading in good yields to the corresponding anellated steroidal 2-amino-thiophenes 12-15. Gewald reaction proved to be an excellent method to access heterocyclic steroids.
Full Text:
PDFReferences
- K.M. Elattar, A. El-Mekabaty, Heterocyclic steroids: Efficient routes and biological characteristics of steroidal fuse bicyclic pyrimidines, J. Heterocycl. Chem., 2021. 58, 389-414.
- M.A. Gouda, M.A. Berghot, G.E. El-Ghani, K.M. Elattar, A.E.G.M. Khalil, Chemistry of 2-aminothiophene-3-carboxamide and related compounds, Turk. J. Chem., 2011, 35, 815-837.
- M. Monier, A. El-Mekabaty, K.M. Elattar, Five-membered ring systems with one heteroatiom: synthetic routes, chemical reactivity, and biological properties of furan-carboxamide analogs, Synth. Commun., 2018, 48, 839-875.
- M. Monier, A. El-Mekabaty, D. Abdel-Latif, B.D. Mert, K.M. Elattar, Heterocyclic steroids: efficient routes for the annulation of pentacyclic steroidal pyrimidines, Steroids, 2020, 154, 108548.
- A. Bhalla, P. Saini, S.S. Bari, 2-aminothiophenes: a review on synthetic routes and applications (biological/synthons), Am. J. PharmTech Res., 2017, 7, 57-78.
- K. Bozorov, L.F. Nie, J. Zhao, H.A. Aisa, 2-Aminothiophene scaffolds: Diverse biological and pharmacological attributes in medicinal chemistry, Eur. J. Med. Chem., 2017, 140, 465-493.
- K. Gewald, Methods for the synthesis and reaction of 2-aminothiophenes, Khim. Geterotsikl. Soedin., 1976, 1299.
- K. Gewald, Methods for the synthesis and reaction of 2-aminothiophenes, Pyatichlen. Aromat. Geterotsikly, Riga, 1979, 85-101.
- V.P. Litvinov, Y.A. Sharanin, F.S. Babichev, Cyclization of nitriles as synthetic route to 2- and 3-aminothiophenes, Sulfur Rep., 1986, 6, 97-135.
- C. Paulmier, Synthesis and reactivity of 3-aminothiophenes and 3,4-diaminothiophenes, Sulfur Rep., 1996, 19, 215-284.
- M. Perrissin, Substituted 2-aminothiophenes. Synthesis, structure, and reactivity. Pharmacological results, Bull. Trav. Soc. Pharm. Lyon, 1982, 26, 76-79.
- R. Alivelu, B. Mohanta, Y. Jahnavi, B.U. Kiran, S.K.K. Naresh, Design, synthesis of novel ethylene bridged N-Acyl homoserine lactones as inhibitors of quorum sensing signaling in pathogenic bacteria to prevent biofilm formation, Int. J. Pharm. Biol. Sci., 2021, 11, 56-65.
- V. Duvauchelle, P. Meffre, Z. Benfodda, Recent contribution of medicinally active 2-aminothiophenes: A privileged scaffold for drug discovery, Eur. J. Med. Chem., 2022, 238, 114502.
- Y. Hu, S. Yang, F.B. Shilliday, B.R. Heyde, K.M. Mandrell, R.H. Robins, J. Xie, M.T. Reding, Y. Lai, D.C. Thompson, Novel metabolic bioactivation mechanism for a series of anti-inflammatory agents (2,5-diaminothiophene derivatives) mediated by cytochrome P450 enzymes, Drug Metab. Dispos., 2010, 38, 1522-1531.
- Y. Huang, A. Doemling, The Gewald multi-component reaction, Mol. Diversity, 2011, 15, 3-33.
- J. Hwang, X. Qiu, L. Borgelt, N. Haacke, L. Kanis, S. Petroulia, R. Gasper, D. Schiller, P. Lampe, S. Sievers, J. Imig, P. Wu, Synthesis and evaluation of RNase L-binding 2-aminothiophenes as anticancer agents, Bioorg. Med. Chem., 2022, 58, 116653.
- M.E. Khalifa, W.M. Algothami, Gewald synthesis, antitumor profile and molecular modeling of novel 5-acetyl-4-((4-acetylphenyl)amino)-2-aminothiophene-3-carbonitrile scaffolds, J. Mol. Struct., 2020, 1207.
- R.W. Sabnis, The Gewald reaction in dye chemistry, Color. Technol., 2016, 132, 49-82.
- K. Gewald, Heterocycles from CH-acidic nitriles. VI. Reaction of methylene-active nitriles with mustard oils and sulfur, J. Prakt. Chem. (Leipzig), 1966, 32, 26-30.
- K. Gewald, Heterocycles from CH-acidic nitriles. IX. Reaction of α-hydroxy ketones with malononitrile, Chem. Ber., 1966, 99, 1002-1007.
- K. Gewald, Heterocycles from CH-acidic nitriles. V. Simultaneous action of sulfur and carbon disulfide on methylene-active nitriles, J. Prakt. Chem. (Leipzig), 1966, 31, 214-220.
- K. Gewald, G. Neumann, H. Boettcher, Heterocycles from CH-acidic nitriles. XI. New synthesis of 2-aminothionaphthene, Z. Chem., 1966, 6, 261.
- K. Gewald, E. Schinke, Heterocycles from CH-acidic nitriles. X. Reaction of acetone with cyanoacetic ester and sulfur, Chem. Ber., 1966, 99, 271-275.
- K. Gewald, E. Schinke, H. Boettcher, Heterocycles from CH-acidic nitriles. VIII. 2-Aminothiophenes from methylene-active nitriles, carbonyl compounds, and sulfur, Chem. Ber., 1966, 99, 94-100.
- B. Ganem, Strategies for Innovation in Multicomponent Reaction Design, Acc. Chem. Res., 2009, 42, 463-472.
- B. Jiang, T. Rajale, W. Wever, S.-J. Tu, G. Li, Multi-component reactions for the synthesis of heterocycles, Chem. Asian J., 2010, 5,
-2335.
- C.G. Neochoritis, T. Zhao, A. Doemling, Tetrazoles via Multi-component Reactions, Chem. Rev., 2019, 119, 1970-2042.
- S. Protti, S. Garbarino, D. Ravelli, A. Basso, Photoinduced Multi-component Reactions, Angew. Chem., Int. Ed., 2016, 55, 15476-15484.
- B.H. Rotstein, S. Zaretsky, V. Rai, A.K. Yudin, Small Heterocycles in Multi-component Reactions, Chem. Rev., 2014, 114, 8323-8359.
- E. Ruijter, R. Scheffelaar, R.V.A. Orru, Multicomponent Reaction Design in the Quest for Molecular Complexity and Diversity, Angew. Chem., Int. Ed., 2011, 50, 6234-6246.
- P. Slobbe, E. Ruijter, R.V.A. Orru, Recent applications of multi-component reactions in medicinal chemistry, MedChemComm, 2012, 3, 1189-1218.
- B.B. Toure, D.G. Hall, Natural Product Synthesis Using Multicomponent Reaction Strategies, Chem. Rev., 2009, 109, 4439-4486.
- J.S.B. Forero, J. Jones, Jr., F.M. da Silva, The synthetic potential and chemical aspects of the Gewald reaction: application in the preparation of 2-aminothiophenes and related heterocycles, Curr. Org. Synth., 2013, 10, 347-365.
- Z. Puterova, A. Krutosikova, D. Veghc, Gewald reaction: Synthesis, properties and applications of substituted 2-aminothiophenes, ARKIVOC, 2011, 209-246.
- R.W. Sabnis, The Gewald synthesis, Sulfur Rep., 1994, 16, 1-17.
- R.W. Sabnis, D.W. Rangnekar, N.D. Sonawane, 2-Aminothiophenes by the Gewald reaction, J. Heterocycl. Chem., 1999, 36, 333-345.
- Y.A. Sharanin, V.K. Promonenkov, 2'-Aminoandrost-2-eno[2,3-b]thiophenes, Khim. Geterotsikl. Soedin., 1980, 1564-1565.
- N.R. Mohamed, G.A. Elmegeed, M. Younis, Studies on organophosphorus compounds VII: Transformation of steroidal ketones with Lawesson's reagent into thioxo and heterofused steroids. Results of antimicrobial and antifungal activity, Phosphorus, Sulfur Silicon Relat. Elem., 2003, 178, 2003-2017.
- S. John, A.V. Sorokin, P.D. Thompson, Phytosterols and vascular disease, Curr. Opin. Lipidol., 2007, 18, 35-40.
- F. Marangoni, A. Poli, Phytosterols and cardiovascular health, Pharmacol. Res., 2010, 61, 193-199.
- M.D. Patel, P.D. Thompson, Phytosterols and vascular disease, Atherosclerosis (Amsterdam, Neth.), 2006, 186, 12-19.
- G. Vilahur, S. Ben-Aicha, E. Diaz-Riera, L. Badimon, T. Padro, Phytosterols and Inflammation, Curr. Med. Chem., 2019, 26, 6724-6734.
- F. Blanco-Vaca, L. Cedo, J. Julve, Phytosterols in Cancer: From Molecular Mechanisms to Preventive and Therapeutic Potentials, Curr. Med. Chem., 2019, 26, 6735-6749.
- P.G. Bradford, A.B. Awad, Phytosterols as anticancer compounds, Mol. Nutr. Food Res., 2007, 51, 161-170.
- V.R. Ramprasath, A.B. Awad, Role of phytosterols in cancer prevention and treatment, J. AOAC Int., 2015, 98, 735-738.
- N. Shahzad, W. Khan, S. Md, A. Ali, S.S. Saluja, S. Sharma, F.A. Al-Allaf, Z. Abduljaleel, I.A.A. Ibrahim, A.F. Abdel-Wahab, M.A. Afify, S.S. Al-Ghamdi, Phytosterols as a natural anticancer agent: Current status and future perspective, Biomed. Pharmacother., 2017, 88, 786-794.
- H. Tapiero, D.M. Townsend, K.D. Tew, Phytosterols in the prevention of human pathologies, Biomed. Pharmacother., 2003, 57, 321-325.
- T.A. Woyengo, V.R. Ramprasath, P.J.H. Jones, Anticancer effects of phytosterols, Eur. J. Clin. Nutr., 2009, 63, 813-820.
- N.P. Peet, S. Sunder, R.J. Barbuch, A.P. Vinogradoff, Mechanistic observations in the Gewald syntheses of 2-aminothiophenes, J. Heterocycl. Chem., 1986, 23, 129-134.
- D.D. Xuan, Recent Achievement in the Synthesis of Thiophenes, Mini-Rev. Org. Chem., 2021, 18, 110-134.
- M. Carmack, M. Behforouz, G.A. Berchtold, S.M. Berkowitz, D. Wiesler, R. Barone, The Willgerodt-Kindler reactions. 6. Isomerization of the carbonyl group in alkanones and cycloalkanones, J. Heterocycl. Chem., 1989, 26, 1305.
- J.H. Cho, C. Djerassi, Sterols in marine invertebrates. Part 57. Stereostructure, synthesis and acid-catalyzed isomerization of hebesterol, a biosynthetically significant cyclopropyl-containing marine sterol, J. Chem. Soc., Perkin Trans., 1987, 1, 1307-1318.
- B.F. Cravatt, A.H. Lichtman, Fatty acid amide hydrolase: an emerging therapeutic target in the endocannabinoid system, Curr. Opin. Chem. Biol., 2003, 7, 469-475.
- S. Gaetani, V. Cuomo, D. Piomelli, Anandamide hydrolysis: a new target for anti-anxiety drugs ?, Trends Mol. Med., 2003, 9, 474-478.
- S. Kathuria, S. Gaetani, D. Fegley, F. Valino,
A. Duranti, A. Tontini, M. Mor, G. Tarzia, G. La Rana, A. Calignano, A. Giustino, M. Tattoli, M. Palmery, V. Cuomo, D. Piomelli, Modulation of anxiety through blockade of anandamide hydrolysis, Nature Med., 2003, 9, 76-81.
- S.G. Kinsey, S.T. O'Neal, J.Z. Long, B.F. Cravatt, A.H. Lichtman, Inhibition of endocannabinoid catabolic enzymes elicits anxiolytic-like effects in the marble burying assay, Pharmacol., Biochem. Behav., 2011, 98, 21-27.
- M. Maccarrone, A. Finazzi-Agro, Anandamide hydrolase: a guardian angel of human reproduction ?, Trends Pharmacol. Sci., 2004, 25, 353-357.
- J. Cai, F.E. Cooke, B.S. Sherborne, Antagonists of the orexin receptors, Expert Opin. Ther. Pat., 2006, 16, 631-646.
- J. Gatfield, C. Brisbare-Roch, F. Jenck, C. Boss, Orexin Receptor Antagonists: A New Concept In CNS Disorders? ChemMedChem, 2010, 5,
-1214.
- A.L. Gotter, A.J. Roecker, R. Hargreaves, P.J. Coleman, C.J. Winrow, J.J. Renger, Orexin receptors as therapeutic drug targets, Prog. Brain Res., 2012, 198, 163-188.
- T.P. Lebold, P. Bonaventure, B.T. Shireman, Selective orexin receptor antagonists, Bioorg. Med. Chem. Lett., 2013, 23, 4761-4769.
- L. Lin, J. Faraco, R. Li, H. Kadotani, W. Rogers, X. Lin, X. Qiu, P.J. De Jong, S. Nishino, E. Mignot, The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene, Cell (Cambridge, Mass.), 1999, 98, 365-376.
- T.E. Scammell, C.J. Winrow, Orexin receptors: pharmacology and therapeutic opportunities, Annu. Rev. Pharmacol. Toxicol., 2011, 51,
-266.
- C.J. Winrow, J.J. Renger, Discovery and development of orexin receptor antagonists as therapeutics for insomnia, Br. J. Pharmacol., 2014, 171, 283-293.
- M. Sridhar, R.M. Rao, N.H.K. Baba, R.M. Kumbhare, Microwave-accelerated Gewald reaction. Synthesis of 2-aminothiophenes, Tetrahedron Lett., 2007, 48, 3171-3172.
- M. Perrissin, M. Favre, C. Luu-Duc, F. Bakri-Logeais, F. Huguet, G. Narcisse, Thieno[2.3-d]-4-pyrimidones: synthesis, structure and pharmacological properties, Eur. J. Med. Chem.--Chim. Ther., 1984, 19, 420-424.
- Y.W. Dong, X. Jiang, T. Liu, Y. Ling, Q. Yang, L. Zhang, X.K. He, Structure-Based Virtual Screening, Compound Synthesis, and Bioassay for the Design of Chitinase Inhibitors, J. Agr. Food Chem., 2018, 66, 3351-3357.
- H. Mora-Rado, L. Bialy, W. Czechtizky, M. Mendez, J.P.A. Harrity, An Alkyne Diboration/6π-Electrocyclization Strategy for the Synthesis of Pyridine Boronic Acid Derivatives, Angew. Chem., Int. Ed., 2016, 55, 5834-5836.
- Y. Arai, N. Koide, F. Ohki, H. Ageta, L.L. Yang, K.Y. Yen, Fern constituents: triterpenoids isolated from leaflets of Cyathea spinulosa, Chem. Pharm. Bull., 1994, 42, 228-232.
- R.A. Abramovitch, R.G. Micetich, Extractives from Populus tremuloides heartwood--structure and synthesis of tremulone, Can. J. Chem., 1962, 40, 2017-2022.
DOI: http://dx.doi.org/10.13171/mjc02209261446csuk
Refbacks
- There are currently no refbacks.
Copyright (c) 2022 Mediterranean Journal of Chemistry
