Temperature effect on zinc electrodeposition in choline chloride-urea deep eutectic solvent
Abstract
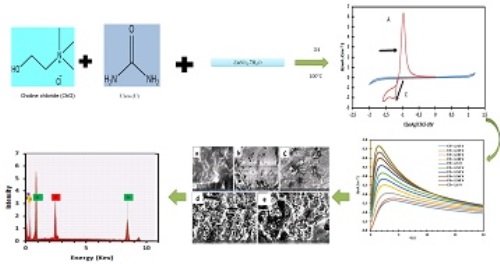
Zinc electrochemistry was studied on a glassy carbon electrode in choline chloride-urea at 100°C. The cyclic voltammetry results show that reducing Zn(II) to Zn (0) is an irreversible process controlled by diffusion. The voltammogram recorded on GC shows an anodic peak at 0.9 V and a cathodic peak at -1.18 V, corresponding to the oxidation of Zn and its reduction. The voltammogram shows a loop characteristic of a nucleation and growth phenomenon reflected by slow deposition kinetics. Chronoamperometric analysis confirms that Zn electrodeposition occurs following instantaneous three-dimensional (3D) nucleation and growth phenomenon. The diffusion coefficient determined using the Cottrell model is 1,9x10-6.cm2.s-1. The deposit’s surface morphology study by scanning electron microscope shows that they are amorphous at 70°C, whereas between 100°C and 110°C, the deposits are very crystalline. EDX analysis reveals the characteristic peaks of Zinc, highlighting deposits made up of Zinc.
Full Text:
PDFReferences
F. Porter, Corrosion Resistance of Zinc and Zinc Alloys, Corrosion Technology, 1st Edition 1994, EBook Published 2014. EBook ISBN 9780429080746. https://doi.org/10.1201/9781482293524.
Z. Liu, T. Cui, G. Pulletikurthi, A. Lahiri, F. Endres, A Prussian Blue/Zinc Secondary Battery with a Bio-Ionic Liquid-Water Mixture as Electrolyte, American Chemical Society Applied Materials and Interfaces, 2016, 8, 12158-12164. https://doi.org/10.1021/acsami.6b01592.
J . Roylance, K-S. Choi, Electrochemical Reductive Biomass Conversion: Direct Conversion of 5-Hydroxymethylfurfural (HMF) to 2,5-Hexanedione (HD) via Reductive Ring-Opening, Green Chemistry, 2016, 10, 2956-2960. https://doi.org/10.1039/C6GC00533K.
H. Liu, S. Szunerits, W. Xu, R. Boukherroub, Preparation of Superhydrophobic Coatings on Zinc as Effective Corrosion Barriers, American Chemical Society Applied Materials and Interfaces, 2009, 1, 1150-1153. https://doi.org/10.1021/am900100q.
K. Maniam, S. Paul, Corrosion Performance of Electrodeposited Zinc and Zinc-Alloy, Coatings in Marine Environment Corrosion Material Degradation, 2021, 2, 163-189. https://doi.org/10.3390/cmd2020010.
E. L. de Araújo, A. M. Rodrigues, A. B. Viana, R. B. Viana, The Influence of Glycerol as an Additive in Zinc-Manganese Alloy Coatings Formed by Electrodeposition, Acta Scientiarum Technology, 2019, 41, E41103. ISSN online: 1807-8664. http://dx.doi.org/10.4025/actascitechnol.v41i1.4110.
J. L. Ortiz-Aparicio, Y. Meas, G. Trejo, R. Ortega, T. W. Chapman, E. Chainet, Effects of Organic Additives on Zinc Electrodeposition from Alkaline Electrolytes, Journal of Applied Electrochemistry, 2013, 43, 289-300. DOI: 10.1007/S10800-012-0518x.
Q. Rayée, T. Doneux, C. Buess-Herman, Underpotential Deposition of Silver on Gold from Deep Eutectic Electrolytes, Electrochimica Acta, 2017, 237, 127-132. https://doi.org/10.1016/j.electacta.2017.03.182.
A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed, V. Tambyrajah, Novel Solvent Properties of Choline Chloride/Urea Mixtures, Chemical Communications, 2003, 1, 70-71. https://Doi.Org/10.1039/B210714G.
E. L. Smith, A. P. Abbott, K. S. Ryder, Deep Eutectic Solvents and Their Applications, Chemical Review, 2014, 114, 11060-11082. Https://Doi.Org/10.1021/Cr300162p.’
F. Ilgen, D. Ott, D. Kralisch, C. Reil, A. Palmberger, B. König, Conversion of Carbohydrates into 5-Hydroxymethylfurfural in Highly Concentrated Low Melting Mixtures, Green Chemistry, 2009, 11, 1948-1954. https://Doi.Org/10.1039/B917548M.’
R. Bomparola, S. Caporali, A. Lavacchi, U. Bardi, Silver Electrodeposition from Air and Water-Stable Ionic Liquid: An Environmentally Friendly Alternative to Cyanide Baths, Surface Coatings Technology, 2007, 24, 9485-9490. Https://Doi.Org/10.1016/j.Surfcoat.2007.04.008’
A. P. Abbott, J.C. Barron, G. Frisch, S. Gurman, K. S. Ryder and A. F. Silva, Double Layer Effects on Metal Nucleation in Deep Eutectic Solvents, Phys. Chem. Chem. Phys,2011,13,10224–10231’.
L.Vieira, R.Schennach, B.Gollas, The Effect of the Electrode Material on the Electrodeposition of Zinc from Deep Eutectic Solvents, Electro-chimica Acta 2015, http://Dx.Doi.Org/10.1016/j.Electacta.2015.11.030.
E. Emanuele, A. Li Bassi, A. Macrelli, C. Mele, J. Strada, B. Bozzini, Zinc Electrode Cycling in Deep Eutectic Solvent Electrolytes: An Electrochemical Study. Molecules 2023, 28, 957. https://Doi.Org/ 10.3390/Molecules28030957).
J. Chen, M. Zhu, M. Gan, X. Wang, C. Gu, J. Tu, Rapid Electrodeposition and Corrosion Behavior of Zn Coating from a Designed Deep Eutectic Solvent. Metals 2023, 13, 172. https://Doi.Org/ 10.3390/Met13010172.).
C. Xiao, X. Cunying, X. Qinqin, W. Shuxian, L. Jianru, H. Yixin, The Effect of the Zinc Salt on the Electrochemical Behaviors of Zn in ChCl‑urea Deep Eutectic Solvent, Journal of Solid State Electrochemistry, Ionics 2023, 29 :1255–1265, https://Doi.Org/10.1007/S11581-023-04879-2).
X.L. Zhu, C.Y. Xu, J. Tang, Y.X. Hua, Q.B. Zhang, H. Liu, X. Wang, M.T. Huang, Selective Recovery of Zinc from Zinc Oxide Dust Using Choline Chloride Based Deep Eutectic Solvents, Transactions of. Nonferrous Metals Society,2019 29, 2222−2228.
Q. Xiang, · S. Wang, J. Li, Y. Hua, The Effect of the Zinc Salt on the Electrochemical Behaviors of Zn in ChCl‑urea Deep Eutectic Solvent, Journal of Solid State Electrochemistry, Ionics 2023, 29 :1255–1265, ttps://Doi.Org/10.1007/S11581-023-04879-2.
H. Yang, R. Reddy, Electrochemical Deposition of Zinc from Zinc Oxide in 2 :1 Urea/Choline Chloride Ionic Liquid, Electrochimica Acta, 2014, 147, 513-519. https://Doi.Org/10.1016/j.Electacta.2014.09.137.
X. Xueliang ; Z. Xingli ; L. Xionggang, L. Changyuan, C. Hongwei, X. Qian, Z. Zhongfu, Electrodeposition of Zn and CuZn alloy from ZnO/CuO precursors in a deep eutectic solvent, Applied Surface Science, 2016 385, 481-489. https://doi.org/10.1016/j.apsusc.(2016).05.138))
M. Harati, D. Love, W. Lau, Z. Ding, Preparation of Crystalline Zinc Oxide Films by One-Step Electrodeposition in Reline, Materials Letters, 2012, 89, 339-342. http://dx.doi.org/10.1016/j.matlet.2012.08.136.
H. F. Alesary, S. Cihangir, A. D. Ballantyne, R. C. Harris, D. P. Weston, A. P. Abbott, K. S. Ryder, Influence of Additives on the Electrodeposition of Zinc from a Deep Eutectic Solvent, Electrochimica Acta, 2019, 304, 118-130. https://Doi.Org/10.1016/j.Electacta.2019.02.090.
S. Ganesan, P. Ganesan, B..N. Popov, Electrodeposition and Characterization of Zn-Mn Coatings for Corrosion Protection Surface & Coatings Technology, 2014, 238, 143-151.
K. K. Maniam and S. Paul, Progress in Electrodeposition of Zinc and Zinc Nickel Alloys Using Ionic Liquids, Applied Sciences, 2020, 10, 5321. Doi:10.3390/app10155321.
A. A. Ortiz Verdín, R. O. Borges, G. T. Cordova, Y. M. Vong, Electrodeposition of Ni-Rich Alloys from an Acidic Deposition Solution by a Normal Codeposition Mechanism, Electrochemical and Solid-State Letters, 2011, 14, 72-75. http://dx.doi.org/10.1149/1.3567029.
A. El Fazazi, M. Ouakki, M. Cherkaoui, Electrochemical Deposition of Zinc on Mild Steel, Mediterranean Journal of Chemistry, 2019, 8, 30-41. https://doi.org/10.13171/mjc8119021318mo.
D. Sylla, C. Savall, M. Gadouleau, C. Rebere, J. Creus, Ph. Refait, Electrodeposition of Zn–Mn Alloys in Acidic and Alkaline Baths. Influence of Additives on the Morphological and Structural Properties, Journal of Applied Electrochemistry, 2005, 200, 2137-2145.
https://doi.org/10.1016/j.surfcoat.2004.11.020.
G. Deng, Q. Zeng, J. Huang, Electrodeposition Behaviors of Zn-Ni Alloy on Copper Foil with Carrier, 5th International Conference on Advanced Engineering Materials and Technology, 2015, 423-428.
http://dx.doi.org/10.2991/icaemt-15.2015.83.
A. Redjechta, K. Loucif, L. Mentar, M. R. Khelladi, A. Beniaiche, Electrodeposition and Characterization of Cu-Zn Alloy Films Obtained from a Sulfate Bath, Materials and Technologies, 2014, 48, 221-226. ISSN 1580-294.
J. B. Bajat, V. B. Mišković-Stanković, J. P. Popić, D. M. Dražić, Adhesion Characteristics and Corrosion Stability of Epoxy Coatings Electrodeposited on Phosphate Hot-Dip Galvanized Steel, Journal of Serbian Chemical Society, 2008, 63, 201-208. https://Doi.Org/10.1016/j.Porgcoat.2008.06.002.
Po‐Yu Chen, M.‐C. Lin, I‐W. Sun, Electrodeposition of Cu-Zn Alloy from a Lewis Acidic ZnCl2-EMIC Molten Salt, Journal of The Electrochemical Society, 2000, 147, 3350-3355. https://doi.org/10.1149/1.1393905.
Y. Wang, H. Yeh, Y.Tang, C. Kao, P. Chen, Voltammetric Study and Electrodeposition of Zinc in Hydrophobic Room-Temperature Ionic Liquid 1-Butyl-1-Methylpyrrolidinium Bis((Trifluoromethyl)Sulfonyl) Imide ([BMP][TFSI]): A Comparison between Chloride and TFSI Salts of Zinc, Journal of The Electrochemical Society, 2017, 164, 39-47. https://Doi.Org/10.1149/2.0451702JES.’
A. Maciej, N. Łatanik, M. Sowa, I. Matuła, W. Simka, Electrodeposition of Copper and Brass Coatings with Olive-Like Structure, Chemistry and Materials Science, 2021, 14, 1762-1765. https://doi.org/10.3390/ma14071762.
W. He, L. Shen, Z. Shi, B. Gao, X. Hu, J. Xu, Z. Wang, Zinc Electrodeposition from Zinc Oxidein the Urea/1-Ethyl-3-Methylimidazolium Chloride at 353 K, Electrochemistry-Tokyo, 2016, 84, 872-877. http://Dx.Doi.Org/10.5796/Electrochemistry.84.872.
J. C. Myland, K. B. Oldham, Cottrell’s Equation Revisited: An Intuitive, but Unreliable, Novel Approach to the Tracking of Electrochemical Diffusion, Electrochemical Communication, 2004, 6, 344-350.
https://doi.org/10.1016/j.elecom.2004.01.013.
L. Simanavicius, A. Stakenas, A Sarkis, the Initial Stages of Aluminum and Zinc Electrodeposition from an Aluminum Electrolyte Containing Quaternary Aralkylammonium Compound, Electrochimica Acta, 1997, 42, 1581-1586. https://doi.org/10.1016/S0013-4686(96)00319-2.
J. Depoorter, X. Yan, B. Zhang, G. Sudre, A. Charlot, E. Fleury and J. Bernard, All Poly(Ionic Liquid) Block Copolymer Nanoparticles from Antagonistic Isomeric Macromolecular Blocks via Aqueous RAFT Polymerization-Induced Self-Assembly, Polymer Chemistry, 2021, 12, 82-91. https://doi.org/10.1039/D0PY00698J.
B. Scharifker, G. Hills, Theoretical and Experimental Studies of Multiple Nucleation, Electrochemical Acta, 1983, 28, 879-889. https://doi.org/10.1016/0013-4686(83)85163-9.
B. Scharifker, J. Mostany, Three-Dimensional Nucleation with Diffusion Controlled Growth, Journal of Electroanalytical Chemistry Interfacial Electrochemistry, 1984, 177, 13-23. https://doi.org/10.1016/0022-0728(84)80207-7.
M. S. Rehbach, J.H.O.J. Wijenberg, E. Bosco, J. H. Sluyters, The Theory of Chronoamperometry for the Investigation of Electrocrystallisation Mathematical Description and Analysis in the Case of Diffusion-Controlled Growth, Journal of Electroanalytical Chemistry, 1987, 236, 1-20. https://doi.org/10.1016/0022-0728(87)88014-2.
L. Heerman, A. Tarallo, Theory of the Chronoamperometric Transient for Electrochemical Nucleation with Diffusion-Controlled Growth, Journal of Electroanalytical Chemistry, 1999, 470, 70-76. https://doi.org/10.1016/S0022-0728(99)00221-1.
P. Allongue, E. Souteyrand, Metal Electrodeposition on Semiconductors: Part 2. Description of the Nucleation Processes, Journal of Electroanalytical Chemistry, 1993,362, 79-87. https://Doi.Org/10.1016/0022-0728(93)80008-6.
DOI: http://dx.doi.org/10.13171/mjc02306161653bougouma
Refbacks
- There are currently no refbacks.
Copyright (c) 2023 Mediterranean Journal of Chemistry
