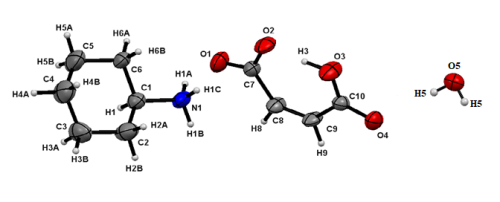
Crystal structure of cyclohexylammonium hydrogen maleate salt hemi-hydrated
Abstract
The compound CyNH3[HOOC(CH)2COO]·H2O has been obtained using one pot-synthesis process by mixing, in methanol, HOOC(CH)2COOH, CyNH2 and SnPh3Cl in a 1:1:1 molar ratio. X-ray diffraction analysis of the compound reveals that in the crystal structure, hydrogen maleate [HOOC(CH)2COO]- in cis configuration interacts with [CyNH3]+ cations and water molecules to generate an R66(22) ring. The interconnections of the rings via simple (N–H··O and O–H··O) and bifurcated (N–H···(O, O)) hydrogen bonds give rise to a 2D supramolecular structure.
Full Text:
PDFReferences
- P. H. Stahl, C. G. Wermuth, Pharmaceutical Salts: Properties, Selection and Use, John Wiley & Sons, 2002.
- D. Seye, A. Toure, M. Lo, C. A. K. Diop, L. Diop, D. Geiger, Crystal Structure of Diisopropylammonium Hydrogen Maleate, Sci. J. Chem., 2019, 7, 110-113.
- C. C. Silva, M. L. Cirqueira, F. T. Martis, Lamivudine salts with 1, 2-dicarboxylic acids: A new and a rare synthon with double pairing motif fine-tuning their solubility, CrystEngComm, 2013, 15, 6311–6317.
- L. Orola, I. Sarcevica, A. Kons, A. Actins, M. V. Veidis, Conformation of the umifenovir cation in the molecular and crystal structures of four carboxylic acid salts, J. Mol. Struct., 2014, 1056, 63–69.
- J. M. Chen, Z. Z. Wang, C. B. Wu, S. Li, T. B. Lu, Crystal engineering approach to improve the solubility of mebendazole, Cryst EngComm., 2012, 14, 6221–6229.
- S. Jayanty, T. Akutagawa, T. Nakamura, Hydrogen-bonded maleate counter anions in tetrathiafulvalene crystals, CrystEngComm., 2012, 14, 3875–3881.
- G. T. Gál, N. V. May, P. Bombicz, Acta Cryst, 2016, E72, 72, 612–615.
- Y-F. Zhang, X-Y. Chen, X-J. Dai, Y-N. Zhang, H-L. Yu, D-F. Zhong, Influence of omeprazole on pharmacokinetics of domperidone given as free base and maleate salt in healthy Chinese patients, Acta Pharmacologica Sinica., 2007, 28, 1243-1246.
- A. E. Till, H. J. Gomez, M. Hichens, A. J. Bolognese, W. R. McNabb, B. A. Brooks, F. Noormohamed, A. F. Lant, Pharmacokinetics of repeated single oral doses of enalapril maleate (MK‐421) in normal volunteers, Biopharmaceutics and drugs Disposition, 1984, 5, 3, 273-280.
- P. A. Meredith, Potential concerns about generic substitution: bioequivalence versus therapeutic equivalence of different amlodipine salt forms, Current Medical Research and Opinion,2009, 25, 9, 2179-2189.
- D. Madsen, C. Flensburg, S. Larsen, Properties of the Experimental Crystal Charge Density of Methylammonium Hydrogen Maleate. A Salt with a Very Short Intramolecular O− H− O Hydrogen Bond, Phys. Chem. A., 1998, 102, 12, 2177–2188.
- T. Poręba, P. Macchi, N. Casati, Structural Variety of Alkali Hydrogen Maleates at High Pressure, Cryst. Growth Des., 2020, 20, 7, 4375–4386.
- Agilent CrysAlis PRO. Agilent Technologies, Yarnton, England, 2014.
- A. Altomare, G. Cascarano, C. Giacovazzo, A. Guagliardi, M. C. Burla, G. Polidori, M. Camalli, SIRPOW. 92–a program for automatic solution of crystal structures by direct methods optimized for powder data, J. Appl. Cryst., 1992, 27, 435-436.
- G. M. Sheldrick, A magic triangle for experimental phasing of macromolecules, Acta Cryst., 2008, A64, 112–122.
- C. S. D, Mercury, 2.0: CF Macrae, IJ Bruno, JA Chisholm, PR Edgington, P. McCabe, E. Pidcock, L. Rodriguez-Monge, R. Taylor, J. van de Streek and PA Wood, J. Appl. Cryst., 2008, 41, 466–470.
- L. J. Farrugia, WinGX and ORTEP for Windows: an update, J. Appl. Cryst., 2012, 45, 849–854.
- A. L. Spek, Structure validation in chemical crystallography, Acta Cryst., 2009, D65, 148–155.
- M. Zeller, P. Smith, D. K. Purcell, M. Okezue, D. T. Smith, S. R. Byrnh, K. L. Clasee, Maleate salts of bedaquiline, Acta Cryst., 2021, E77, 433–445.
- B. Sarr, A. Mbaye, C. A. K. Diop, F. Melin, P. Hellwig, M. Sidibé, Y. Rousselin, Crystal structure of bis (diisopropylammonium) molybdate, Acta Cryst., 2018, E74, 1682–1685.
- G. Radha, B. V. Pandiyan, P. Deepa, S. Govindarajan, P. Kolandaivel, D. Nataraj, Synthesis and Experimental Studies on Supramolecular Synthons of Aminoguanidinium Carboxylates: A Case Study of π‐HoleBonded Carbon Bonding via Theoretical Approaches, Chemistry Select., 2018, 3, 10032–10048.
DOI: http://dx.doi.org/10.13171/mjc02301041662diallo
Refbacks
- There are currently no refbacks.
Copyright (c) Mediterranean Journal of Chemistry
