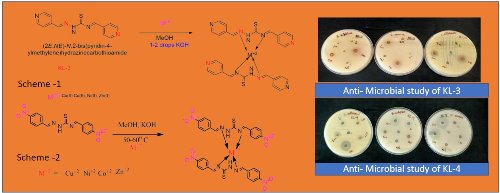
Molecular Docking and Biological Activity of Pyridine and Thiosemicarbazide derived Schiff base Ligands
Abstract
Full Text:
PDFReferences
-NE Borisova, MD. Reshetova, Y.A. Ustynyuk, Metal-Free Methods in the Synthesis of Macrocyclic Schiff Bases, Chem. Rev., 2007, 107 (1), 46–79.
-C. Jiang, Z. Song, L. Yu, S. Ye, H. He, Fluorescent Probes Based on Macrocyclic Hosts: Construction, Mechanism and Analytical Applications, TrAC Trends Anal. Chem., 2020, 133, 116086.
-A.J. Gallant, M.J. MacLachlan, Ion‐induced Tubular Assembly of Conjugated Schiff‐base Macrocycles, Angew. Chemie, 2003, 115 (43), 5465–5468.
-S. Tariq, P. Kamboj, M. Amir, Therapeutic Advancement of Benzothiazole Derivatives in the Last Decennial Period, Arch. Pharm., 2019 352 (1), 1800170.
- B. Vavaiya, S. Patel, V. Pansuriya, V. Marvaniya, P. Patel, Synthesis, Antitubercular Evaluation and Molecular Docking Studies of Nitrogen-Rich Piperazine-Pyrimidine-Pyrazole Hybrid Motifs, Curr. Chem. Lett., 2022, 11 (1), 95–104.
- P.A. Vigato, S. Tamburini, L. Bertolo, The Development of Compartmental Macrocyclic Schiff Bases and Related Polyamine Derivatives, Coord. Chem. Rev., 2007, 251 (11–12), 1311–1492.
- G. Yakalı, Examination of Aggregation-Induced Enhanced Emission in a Propeller-Shaped Chiral Non-Conjugated Blue Emitter from Restricted Intramolecular Rotation and J-Type Π π Stacking Interactions, Phys. Chem. Chem. Phys., 2021, 23 (19), 11388–11399.
-K. Amimoto, T. Kawato, Photochromism of Organic Compounds in the Crystal State J. Photochem. Photobiol. C Photochem. Rev., 2005, 6 (4), 207–226.
- A.B. Deshmukh, R. Sharma, P. Jain, P.K. Upadhyay, P.P. Timbadia, V. Kshirsagar, M.V. Bramhe, M.V. Sarode, L.G. Malik, R.K. Watile, Engineering and Technology (ET), Recent Advances in Science, Management, Engineering and Technology, 2015.
-AL Berhanu, I. Mohiuddin, A.K. Malik, J.S. Aulakh, V. Kumar, K.H. Kim, A Review of the Applications of Schiff Bases as Optical Chemical Sensors, TrAC Trends Anal. Chem., 2019, 116, 74–91.
-M.T. Ali, N. Blicharska, J.A. Shilpi, V. Seidel, Investigation of the Anti-TB Potential of Selected Propolis Constituents Using a Molecular Docking Approach, Sci. Rep., 2018, 8 (1), 1–8.
DOI: http://dx.doi.org/10.13171/mjc02309141705chourasia
Refbacks
- There are currently no refbacks.
Copyright (c) 2023 Mediterranean Journal of Chemistry
