In search of cytotoxic abietyl amides
Abstract
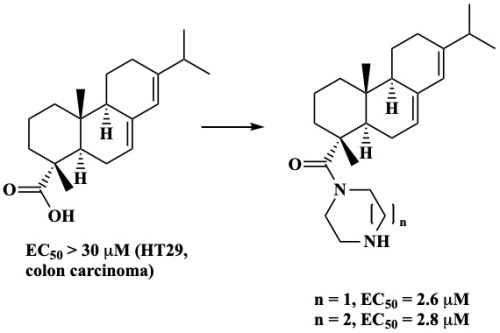
Based on prior research findings with pentacyclic triterpenoids, it was hypothesized that (un)-substituted benzylamides would exhibit enhanced cytotoxic activity compared to parent abietic acid. Conversely, none of these compounds was cytotoxic, but (homo)-piperazinyl amides demonstrated significant cytotoxic activity across multiple cell lines, even at concentrations as low single-digit micromolar levels. Additional staining experiments revealed that the most potent compound (with an EC50 value of 2.8 mM for HT29 colon carcinoma cells) primarily induced apoptosis rather than necrosis.
Full Text:
PDFReferences
- G. Bonsignore, M. Patrone, F. Grosso, S. Martinotti, E. Ranzato, Cancer Therapy Challenge: It Is Time to Look in the "St. Patrick's Well" of the Nature, Int. J. Mol. Sci., 2021, 22, 10380.
- S. Coseri, Natural products and their analogues as efficient anticancer drugs, Mini-Rev. Med. Chem., 2009, 9, 560-571.
- M. Erkisa, M. Sariman, O.G. Geyik, C. Geyik, T. Stanojkovic, E. Ulukaya, Natural products as a promising therapeutic strategy to target cancer stem cells, Curr. Med. Chem., 2022, 29, 741-783.
- M. He, L. Xia, J. Li, Potential Mechanisms of Plant-Derived Natural Products in the Treatment of Cervical Cancer, Biomolecules, 2021, 11, 1539.
- D.J. Newman, G.M. Cragg, Natural Products as Sources of New Drugs from 1981 to 2014, J. Nat. Prod., 2016, 79, 629-661.
- D.J. Newman, G.M. Cragg, Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019, J. Nat. Prod., 2020, 83, 770-803.
- C. Fedele, R.W. Tothill, G.A. McArthur, Navigating the challenge of tumor heterogeneity in cancer therapy, Cancer Discovery, 2014, 4, 146-148.
- I.I. Wistuba, J.G. Gelovani, J.J. Jacoby, S.E. Davis, R.S. Herbst, Methodological and practical challenges for personalized cancer therapies, Nat. Rev. Clin. Oncol., 2011, 8, 135-141.
- S. Arı, M. Kargıoğlu, M. Temel, M. Konuk, Traditional Tar Production from the Anatolian Black Pine [Pinus nigra Arn. subsp. pallasiana (Lamb.) Holmboe var. pallasiana.- and its usages in Afyonkarahisar, Central Western Turkey,
J Ethnobiol Ethnomed, 2014, 10, 29.
- N. El Omari, F. Ezzahrae Guaouguaou, N. El Menyiy, T. Benali, T. Aanniz, I. Chamkhi,
A. Balahbib, D. Taha, M.A. Shariati, G. Zengin, M. El-Shazly, A. Bouyahya, Phytochemical and biological activities of Pinus halepensis mill., and their ethnomedicinal use, J. Ethnopharmacol., 2021, 268, 113661.
- I. Tumen, E.K. Akkol, H. Tastan, I. Suntar,
M. Kurtca, Research on the antioxidant, wound healing, and anti-inflammatory activities and the phytochemical composition of maritime pine (Pinus pinaster Ait), J. Ethnopharmacol., 2018, 211, 235-246.
- J.R. Hanson, Diterpenoids of terrestrial origin, Nat. Prod. Rep., 2011, 28, 1755-1772.
- P. Reveglia, A. Cimmino, M. Masi, P. Nocera,
N. Berova, G. Ellestad, A. Evidente, Pimarane diterpenes: Natural source, stereochemical configuration, and biological activity, Chirality, 2018, 30, 1115-1134.
- R. Acquaviva, G.A. Malfa, M.R. Loizzo, J. Xiao, S. Bianchi, R. Tundis, Advances on Natural Abietane, Labdane and Clerodane Diterpenes as Anticancer Agents: Sources and Mechanisms of Action, Molecules, 2022, 27, 4791.
- A.H. Conner, B.A. Nagasampagi, J.W. Rowe, Terpenoid and other extractives of western white pine bark, Phytochemistry, 1980, 19, 1121.
- I.I. Bardyshev, Purification of l-abietic acid (low-melting form), Zh. Obshch. Khim., 1941, 11, 996-1000.
- A.T. Karlberg, E. Bergstedt, A. Boman,
K. Bohlinder, C. Liden, J. Lars, G. Nilsson, J.E. Wahlberg, Is abietic acid the allergenic component of colophony?, Contact Dermatitis, 1985, 13, 209.
- A.T. Karlberg, K. Bohlinder, A. Boman,
U. Hacksell, J. Hermansson, S. Jacobsson, J.L.G. Nilsson, Identification of 15-hydroperoxyabietic acid as a contact allergen in Portuguese colophony, J. Pharm. Pharmacol., 1988, 40, 42.
- M.A. Fernandez, M.P. Tornos, M.D. Garcia,
B. De las Heras, A.M. Villar, M.T. Saenz, Anti-inflammatory activity of abietic acid, a diterpene isolated from Pimenta racemosa var. grissea,
J. Pharm. Pharmacol., 2001, 53, 867-872.
- N. Takahashi, T. Kawada, T. Goto, C.-S. Kim,
A. Taimatsu, K. Egawa, T. Yamamoto,
M. Jisaka, K. Nishimura, K. Yokota, R. Yu,
T. Fushiki, Abietic acid activates peroxisome proliferator-activated receptor-γ (PPARγ) in RAW264.7 macrophages and 3T3-L1 adipocytes to regulate gene expression involved in inflammation and lipid metabolism, FEBS Lett., 2003, 550, 190-194.
- M.A. Gonzalez, J. Correa-Royero, L. Agudelo, A. Mesa, L. Betancur-Galvis, Synthesis and biological evaluation of abietic acid derivatives, Eur. J. Med. Chem., 2009, 44, 2468-2472.
- H. Haffez, S. Osman, H.Y. Ebrahim, Z.A. Hassan, Growth Inhibition and Apoptotic Effect of Pine Extract and Abietic Acid on MCF-7 Breast Cancer Cells via Alteration of Multiple Gene Expressions Using In Vitro Approach, Molecules, 2022, 27, 293.
- Y. Ito, T. Ito, K. Yamashiro, F. Mineshiba,
K. Hirai, K. Omori, T. Yamamoto, S. Takashiba, Antimicrobial and antibiofilm effects of abietic acid on cariogenic Streptococcus mutans, Odontology, 2020, 108, 57-65.
- N. Yoshida, T. Takada, Y. Yamamura, I. Adachi, H. Suzuki, J. Kawakami, Inhibitory effects of terpenoids on multidrug resistance-associated protein 2- and breast cancer resistance protein-mediated transport, Drug Metab. Dispos., 2008, 36, 1206-1211.
- B. Brandes, S. Hoenke, N. Starke, I. Serbian,
H.-P. Deigner, A. Al-Harrasi, R. Csuk, Synthesis and cytotoxicity of apoptosis-inducing
N-heterocyclic triterpene amides, Eur. J. Med. Chem. Rep., 2022, 6, 100085.
- N.V. Heise, J. Heisig, L. Hoehlich, S. Hoenke,
R. Csuk, Synthesis and cytotoxicity of diastereomeric benzylamides derived from maslinic acid, augustic acid and bredemolic acid, Results Chem., 2023, 5, 100805.
- S. Hoenke, N.V. Heise, M. Kahnt, H.-P. Deigner, R. Csuk, Betulinic acid-derived amides are highly cytotoxic, apoptotic and selective, Eur. J. Med. Chem., 2020, 207, 112815.
- M. Kozubek, L. Hoehlich, S. Hoenke, H.-P. Deigner, A. Al-Harrasi, R. Csuk, Apoptotic activity of substituted 3-O-acetyl-betulinic acid benzylamides, Eur. J. Med. Chem. Rep., 2021, 3, 100016.
- S. Sommerwerk, L. Heller, J. Kuhfs, R. Csuk, Selective killing of cancer cells with triterpenoic acid amides - The substantial role of an aromatic moiety alignment, Eur. J. Med. Chem., 2016, 122, 452-464.
- O. Kraft, A.-K. Hartmann, S. Hoenke, I. Serbian,
R. Csuk, Madecassic Acid-A New Scaffold for Highly Cytotoxic Agents, Int. J. Mol. Sci., 2022, 23, 4362.
- J. Wiemann, A. Al-Harrasi, R. Csuk, Cytotoxic
Dehydroabietylamine Derived Compounds, Anticancer Agents Med. Chem., 2020, 20,
-1767.
DOI: http://dx.doi.org/10.13171/mjc02307191709csuk
Refbacks
- There are currently no refbacks.
Copyright (c) 2023 Mediterranean Journal of Chemistry
