Determination of palladium by the method of concentration in various objects with the participation of synthetic sorbents
Abstract
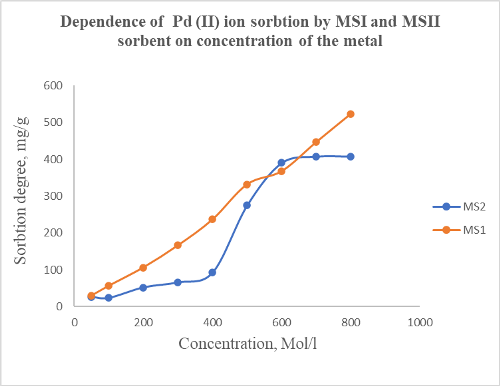
This work developed a method for determining palladium (II) in various objects. For this purpose, new polymer sorbents have been synthesized by chemical modification of a copolymer of styrene and maleic anhydride using formaldehyde and an amine fragment with various functional groups. The resulting polymer sorbents were identified by IR spectroscopy. The temperature stability of the synthesized sorbent with sulfodimezine (MS1) and the amino fragment of 1-aminophenol-2-hydroxy-4-sulfonic acid (MS2), was studied by thermogravimetric analysis. It has been established that sorbents based on a copolymer of maleic anhydride and styrene are unsuitable for use at temperatures above 120°С degrees. The resulting sorbent's sorption and desorption of Pd (II) ion was studied. The sorbent's sorption capacity increases when the density is too high, but the sorbents can be reused for 7-8 cycles. The developed methods make it possible to determine palladium (II) ions by condensation in natural and manufactured objects. Optimal sorption conditions were determined. It has been established that the sorption process must be carried out in an acidic environment. Concentrating palladium (II) ions in various objects using synthesized polymer sorbents has been studied. It has been shown that a more promising method for determining trace amounts of palladium (II) is the photometric method. To carry out photometric studies, it was first investigated with which reagent palladium forms a more stable complex compound. It has been established that palladium forms a more stable complex with 2,2,3- tetrahydroxy-3-sulfonic acid-5-chlorazobenzene. It has been shown that determination reactions based on the formation of various ligand complexes of these reagents have higher performance. It was established that palladium (II) forms stable complexes with reagents of functional groups containing donor atoms S, O, and N. Therefore, the photometric determination used reagents based on pyrogallol. It should be noted that the sorption capacities of complex bonds formed by reagents with donor atoms and palladium ions are high.
Full Text:
PDFReferences
Lozynska, L.V., Tymoshuk, O.S., and Yrublevs’ka, T., “Analysis of multicomponent systems for the contents of iridium and palladium,” Materials Science, vol. 50, no. 6, 2015, pp. 870–876.
Gambarov, D.G., Guseynov, A.G. Synthesis and study of some new monoazo compounds based on pyrogallol, Baku: Scientific works of ser. chemistry, Publishing house of AGU, 1979, 4. p.7-9.
Chatwal, G.R., Sham, Anand Instrumental methods of chemical analysis, G.R. Chatwal, 2011, p.500.
Ermakova, T.G. Sorptive concentration of palladium with a nitrogen-containing heterocyclic copolymer, T.G.Ermakova, L.P.Shaulina, N.P.Kuznecova [et. al.]//, News of the Academy of Sciences. Chemical series, 2017, 6, p.1104-1108.
Korostelev, P.P. Preparation of solutions for chemical analytical work, P.P. Korostelev, Moskow, Chemistry, 1964, p.386.
Nazihah, W., Ibrahim, W., Shamsuddin, M Symmetrical Palladium (II) N, N, O, O-Schiff Base Complex: Efficient Catalyst for Heck and Suzuki Reactions, Crystal structure theory and applications, 2012. vol.1, 3, p. 25-29.
Tarasaevich, B.N. IR spectra of the main classes of organic compounds, B.N.Tarasaevich -Moskow, 2012, p.55.
Moskvin, L.N. Methods of separation and concentration in analytical chemistry. Textbook, L.N. Moskvin, O.V. Rodinkov, Dolgoprudny, 2012, p.352.
Buslaeva, T.M., Ehrlich, G.V., Volchkova, E.V., Mingalev P.G., & Panina N.S., Complexation during Sorption of Palladium (II) Ions by Chemically Modified Silica, Russian Journal of Inorganic Chemistry volume 67, 2022, pp.1191–1202.
Nikolaevich, T.A. Method for leaching palladium from sludge, Patent RU2271399C1 Russian Federation, T.A.Nikolaevich, P.L.Aleekseivich, S.A.Leonidovich [et al.].2006.
Rouessac, F., Rouessac, A. Chemical analysis, Modern instrumentation methods, and techniques, F. Rouessac, A. Rouessac [II title] -England, 2007, p.543.
Maharramova, L.M. Analytical chemistry-2, M.L. Maharramova, S.S.Sultanzade, E.İ. Suleymanova, Y.N. Gahramanli, Baku, 2019, p. 164.
DOI: http://dx.doi.org/10.13171/mjc02401091758hashimova
Refbacks
- There are currently no refbacks.
Copyright (c) 2024 Mediterranean Journal of Chemistry
