Resorcin[4]arene Sulfonic Acid as New and Efficient Organocatalysts for the One Pot Synthesis of Fused Pyrimidine Derivatives
Abstract
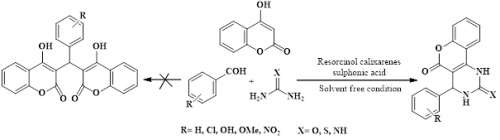
Abstract: One pot and efficient protocol for the synthesis of fused pyrimidine scaffolds has been established using recyclable resorcin[4]arene tetrasulfonic acid as an energetic organo-catalyst under solvent free condition. Resorcin[4]arene tetrasulfonic acid as a recyclable organo-catalyst give excellent yields under solvent free condition for synthesis of fused pyrimidine derivatives. Non-Toxicity, non-chromatographic purification techniques, high yield and recyclable catalyst make the procedure valuable and environmentally friendly.
Full Text:
PDFReferences
H. Bienaymt, C. H. Hulme, G. Oddon and P. Schmitt, Maximizing Synthetic Efficiency: Multi-Component Transformations Lead the Way, Chem. Eur. J., 2000, 6, 3321-3329.
L. Weber, K. Illgen and M. Almstetter, Discovery of New Multi Component Reactions with Combinatorial Methods, Synlett, 1999, 366-374.
R. Armstrong, A. Combs, P. Tempest, S. Brown and T. Keating, Multiple-Component Condensation Strategies for Combinatorial Library Synthesis, Acc. Chem. Res., 1996, 29, 123-131.
A. Domling and I. Ugi, Multicomponent Reactions with Isocyanides, Angew. Chem. Int. Ed., 2000, 39, 3168-3210.
T. Kressman, Ind. Chem. 1960, 3.
S. Shuttleworth, S. Allin and P. Sharma, Functionalised Polymers: Recent Developments and New Applications in Synthetic Organic Chemistry, Synthesis, 1997, 1217-1239.
N. Mizuno and M. Misono, Heterogeneous Catalysis, Chem. Rev. 1998, 98, 199-218.
H. Hattori, Heterogeneous Basic Catalysis, Chem. Rev. 1995, 95, 537-558.
G. Rovnyak, K. Atwal, A. Hedberg, S. Kimball, S. Moreland, J. Gougoutas, B. O’Reilley, J. Schwartz and M. Malley, Dihydropyrimidine calcium channel blockers. 4. Basic 3-substituted-4-aryl-1,4-dihydropyrimidine-5-carboxylic acid esters. Potent antihypertensive agents, J. Med. Chem., 1992, 35, 3254-63.
M. Brands, R. Endermann, R. Gahlmann, J. Krüger and S. Raddatz, Dihydropyrimidinones—a new class of anti-Staphylococcal antibiotics, Bioorg. Med. Chem. Lett., 2003, 13, 241-245.
B. Lagu, D. Tian, G. Chiu, D. Dhanapalan, J. Fang, Q. Shen, C. Forray, R. Ransom, R. Chang, K. Vyas, K. Zhang and C. Gluchowski, Synthesis and evaluation of furo[3,4-d]pyrimidinones as selective α1a-adrenergic receptor antagonists, Bioorg. Med. Chem. Lett., 2000, 10, 175-178.
H. Stefani, C. Oliveira, R. Almeida, C. Pereira, R. Braga, C. Cella, V. Borges, L. Savegnago and C. Nogueira, Dihydropyrimidin-(2H)-ones Obtained by Ultrasound Irradiation: A New Class of Potential Antioxidant Agents, Eur. J. Med.Chem., 2006, 41, 513-518.
C. Kappe, Recent Advances in the Biginelli Dihydropyrimidine Synthesis. New Tricks from an Old Dog, Acc. Chem. Res., 2000, 33, 879-888.
C. Kappe, Biologically Active Dihydropyrimidones of the Biginelli-Type— A Literature Survey, Eur. J. Med. Chem., 2000, 35, 1043-1052.
B. Jauk, T. Pernat and C. Kappe, Design and Synthesis of a Conformationally Rigid Mimic of the Dihydropyrimidine Calcium Channel Modulator SQ 32,926, Molecules, 2000, 5, 227-239.
L. Hench and J. West, The Sol-Gel Process, Chem. Rev., 1990, 90, 33-72.
A. Patil, N. Kumar, W. Kokke, M. Bean, A. Freyer, C. De Brosse, S. Mai, A. Truneh, D. Faulkner, B. Carte, A. Breen, R. Hertzberg, R. Johnson, J. Westley and B. Potts, Novel Alkaloids from the Sponge Batzella sp.: Inhibitors of HIV gp120-Human CD4 Binding J. Org. Chem., 1995, 60, 1182-1188.
K. Mulani, V. Patil, N. Chavan and K. Donde, Adsorptive removal of strontium(II) using macroporous poly(AGE-co-EGDMA) beads modified with resorcin[4]arene, Bulletin of material Science, 2019, 42(2), 82.
S. Shinkai, K. Araki, T. Tsubaki, T. Arimura and O. Manabe, New syntheses of calixarene-p-sulphonates and p-nitrocalixarenes, J. Chem. Soc. Perkin Trans, 1987, I, 2297-2299.
Refbacks
- There are currently no refbacks.
Copyright (c) 2024 Mediterranean Journal of Chemistry
