Theoretical Investigation of Aluminum Corrosion Inhibition Using Chalcone Derivatives
Abstract
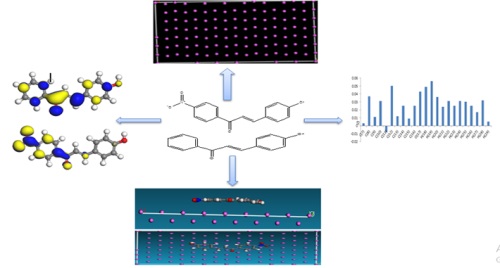
The corrosion of Aluminum is a critical issue in various industrial applications. Using computational methods, this study theoretically investigated the corrosion inhibition properties of two chalcone derivatives. The aim was to understand the molecular mechanisms underlying the inhibitory effect of chalcone derivatives on aluminum corrosion. Density functional theory calculations and molecular dynamics simulations were employed to predict the adsorption behavior and electronic properties associated with the interaction between chalcone derivatives and aluminum surfaces. The binding strength of the inhibitor molecules on aluminum surfaces is of the order HNP > HPP, which agrees with the experimentally determined inhibition efficiencies. Considering those mentioned above, our method will be helpful for rapid quantum chemical calculations and molecular dynamics simulation prediction of a potential inhibitor from many similar inhibitors and then for their logical synthesis for application in corrosion inhibition via a wet chemical synthetic route.
Full Text:
PDFReferences
A. Al-Hashem, M. Lowther, J. Al-Besharah, H.M. Shalaby, Industrial corrosion and corrosion control technology, Kuwait Institute for Scientific Research, 1996, 449-500.
B. Valdez, M. Schorr, R. Zlatev, M. Carrillo, M. Stoytcheva, L. Alvarez, N. Rosas, Corrosion control in industry, Environmental and Industrial Corrosion-Practical and Theoretical Aspects, 2012.
A. Celik-Kucuk, T. Abe, Lithium trifluorosulfonimide salt containing siloxane-based electrolytes for lithium-ion batteries: Aluminum corrosion behaviors and electrochemical properties, Journal of Power Sources, 2023, 556, 232520.
C. L. Li, S.W. Zeng, W. Peng, Z.J. Li, Y. Li, D.N. Zhao, S.Y. Li, Mechanism of aluminum corrosion in LiFSI-based electrolyte at elevated temperatures, Transactions of Nonferrous Metals Society of China, 2021, 31(5), 1439-1451.
A. Celik-Kucuk, T. Abe, Salt-Concentrated Siloxane-Based Electrolytes for Lithium Metal Batteries: Physical Properties, Electrochemical Properties, and Cell Performance, ACS Applied Energy Materials, 2023, 6(9), 4618-4629.
M.A. Wahid, A.N. Siddiquee, Z.A. Khan, Aluminum alloys in marine construction: characteristics, application, and problems from a fabrication viewpoint, Marine Systems & Ocean Technology, 2020, 15, 70-80.
J. Jung, S. Oh, H. Kwon, Effects of environmental factors on corrosion behaviour of aluminum, Materials and Corrosion, 2021, 72(3), 557-563.
M.A.A. Khan, O.M. Irfan, F. Djavanroodi, M. Asad, Development of Sustainable Inhibitors for Corrosion Control, Sustainability, 2022, 14(15), 9502.
W. Mecibah, H. Allal, M. Cherifi, S. Bouasla, B. Nabila, Theoretical study of chalcone derivatives as corrosion inhibitors for Aluminum in acidic environment, Rev. Roum. Chim., 2021, 66(7), 633-644.
A.M. Maharramov, Y.V. Mamedova, M.R. Bayramov, I.G. Mamedov, Chalcone derivatives as corrosion inhibitors for mild steel in brine-kerosene solution, Russian Journal of Physical Chemistry A, 2018, 92, 2154-2158.
S. Velrani, R. Mahalakshmi, Investigation of inhibition effect of naphthyl chalcones on mild steel corrosion in sulphuric acid medium, Rasayan J. Chem., 2015, 8, 156-160.
A.S. Fouda, M. Abdallah, M. Eissa, Corrosion inhibition of Aluminum in 1 M phosphoric acid solutions using some Chalcones derivatives and synergistic action with halide ions, African Journal of Pure and Applied Chemistry, 2013, 7(12), 394-404.
A. Chaouiki, H. Lgaz, R. Salghi, M. Chafiq, H. Oudda, K.S. Bhat, I.M. Chung, Assessing the impact of electron-donating-substituted chalcones on inhibition of mild steel corrosion in HCl solution: Experimental results and molecular-level insights, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020, 588, 124366.
A.S. Fouda, A.M. Eldesoky, A.F. Hassan, A. Abdelhakim, Performance and Theoretical Study on Corrosion Inhibition of 304 SS in Hydrochloric Acid Solutions by Chalcone Compounds, International Journal, 2014, 2(3), 280-300.
M.Hrimla, L. Bahsis, M.R. Laamari, M. Julve, S.E. Stiriba, An overview on the performance of 1, 2, 3-triazole derivatives as corrosion inhibitors for metal surfaces, International Journal of Molecular Sciences, 2021, 23(1), 16.
H. Lgaz, K.S. Bhat, R. Salghi, S. Jodeh, M. Algarra, B. Hammouti, A. Essamri, Insights into corrosion inhibition behavior of three chalcone derivatives for mild steel in hydrochloric acid solution, Journal of Molecular Liquids, 2017, 238, 71-83.
T.A. Nyijime, H.F. Chahul, A.M. AYuba, F. Iorhuna, Theoretical Investigations on Thiadiazole Derivatives as Corrosion Inhibitors on Mild Steel. Advanced Journal of Chemistry-Section A, 2023, 6(2), 141-154.
S.K. Saha, M. Murmu, N.C. Murmu, I.B. Obot, P. Banerjee, Molecular level insights for the corrosion inhibition effectiveness of three amine derivatives on the carbon steel surface in the adverse medium: a combined density functional theory and molecular dynamics simulation study, Surfaces and interfaces, 2018, 10, 65-73.
R.G. Parr, W. Yang, Density-functional theory of the electronic structure of molecules, Annual review of physical chemistry, 1995, 46(1),
-728.
K.F. Khaled, Studies of iron corrosion inhibition using chemical, electrochemical and computer simulation techniques, Electrochimica Acta, 2010, 55(22), 6523-6532.
H. Lgaz, S. Masroor, M. Chafiq, M. Damej, A. Brahmia, R. Salghi, I.M. Chung, Evaluation of 2-mercaptobenzimidazole derivatives as corrosion inhibitors for mild steel in hydrochloric acid, Metals, 2020, 10(3), 357.
S.K. Saha, A. Hens, N.C. Murmu, P. Banerjee, A comparative density functional theory and molecular dynamics simulation studies of the corrosion inhibitory action of two novel N-heterocyclic organic compounds along with a few others over steel surface, Journal of Molecular Liquids, 2016, 215, 486-495.
A. Singh, K.R. Ansari, P. Banerjee, M. Murmu, M.A. Quraishi, Y. Lin, Corrosion inhibition behavior of piperidinium based ionic liquids on Q235 steel in hydrochloric acid solution: experimental, density functional theory and molecular dynamics study, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 623, 126708.
P. Udhayakalaa, T.V. Rajendiranb, S. Gunasekaran, Theoretical study using DFT calculations on inhibitory action of some pyrazole derivatives on steel, Journal of Advanced Scientific Research, 2013, 4(02), 31-37.
K.F. Khaled, M.M. Al-Qahtani, The inhibitive effect of some tetrazole derivatives towards Al corrosion in acid solution: Chemical, electrochemical and theoretical studies, Materials Chemistry and Physics, 2009, 113(1), 150-158.
A.M. Ayuba, T.A Nyijime, Theoretical study of 2-methyl benzoazole and its derivatives as corrosion inhibitors on aluminium metal surface, Journal of Applied Science and Environmental Studies, 2021, 4(2), 4-2.
A. Popova, M. Christov, T. Deligeorgiev, Influence of the molecular structure on the inhibitor properties of benzimidazole derivatives on mild steel corrosion in 1 M hydrochloric acid, corrosion, 2003.
E.E. Ebenso, P.C. Okafor, U.J. Ekpe, Studies on the inhibition of aluminum corrosion by 2-acetylphenothiazine in chloroacetic acids, Anti-Corrosion Methods & Materials, 2003, 50, 414-421.
E. McCafferty, H. Leidheiser, Corrosion Control by Coating, Science Press, Princeton, 1979.
S.A. Umoren, E.E. Ebenso, The synergistic effect of polyacrylamide and iodide ions on the corrosion inhibition of mild steel in H2SO4, Mater. Chem. Phys., 2007, 106, 387-393.
T.A. Nyijime, F. Iorhuna, Theoretical study of 3-(4-hydroxyphenyl)-1-(4-nitrophenyl) prop-2-en-1-one and 3-(4-hydroxyphenyl)-1-phenylprop-2-en-1-one as corrosion inhibitors on mild steel, Applied Journal of Environmental Engineering Science, 2022, 8(2), 2-8.
A.D. Becke, Density‐functional thermochemistry, The effect of the exchange‐only gradient correction, The Journal of Chemical Physics, 1992, 96(3), 2155-2160.
T.A. Salman, K.A. Samawi, J.K. Shneine, Electrochemical and computational studies for mild steel corrosion inhibition by Benzaldehydethiosemicarbazone in acidic medium, Portugaliae Electrochimica Acta, 2019, 37(4), 241-255.
C. Lee, W. Yang, R.G. Parr, Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density, Physical Review B, 1988, 37(2), 785.
T.A. Nyijime, H.F. Chahul, A.M. Ayuba, F. Iorhuna, Theoretical investigations on thiadiazole derivatives as corrosion inhibitors on mild steel, Advanced Journal of Chemistry-Section A, 2023, 6, 141.
N.O. Obi-Egbedi, I.B. Obot, M.I. El-Khaiary, S.A. Umoren, E.E. Ebenso, Computational simulation and statistical analysis on the relationship between corrosion inhibition efficiency and molecular structure of some phenanthroline derivatives on mild steel surface, Int. J. Electrochem. Sci., 2011, 6(1), 5649-5675.
C.B. Verma, M.A. Quraishi, A. Singh, 2-Aminobenzene-1, 3-dicarbonitriles as green corrosion inhibitor for mild steel in 1 M HCl: Electrochemical, thermodynamic, surface and quantum chemical investigation, Journal of the Taiwan Institute of Chemical Engineers, 2015, 49, 229-239.
R. Kinkar Roy, K. Hirao, S. Krishnamurthy, S. Pal, Mulliken population analysis based evaluation of condensed Fukui function indices using fractional molecular charge, The Journal of Chemical Physics, 2001, 15(7), 2901-2907.
H.H. Zhang, Y. Chen, Z. Zhang, Comparative studies of two benzaldehyde thiosemicarbazone derivatives as corrosion inhibitors for mild steel in 1.0 M HCl, Results in Physics, 2018, 11, 554-563.
R. Pal, P.K. Chattaraj, Electrophilicity index revisited, Journal of Computational Chemistry, 2023, 44(3), 278-297.
H. Zou, L.J. Arachchige, W. Rong, C. Tang, R. Wang, S. Tan, L. Duan, Low‐Valence Metal Single Atoms on Graphdiyne Promotes Electrochemical Nitrogen Reduction via M‐to‐N2 π‐Back donation, Advanced Functional Materials, 2022, 32(24), 2200333.
E.S.M. Sherif, Effects of 2-amino-5-(ethylthio)-1,3,4-thiadiazole on copper corrosion as a corrosion inhibitor in 3% NaCl solutions, Appl. Surf. Sci., 2006, 252, 8615-8623.
L. Guo, M. Zhu, J. Chang, R. Thomas, R. Zhang, P. Wang, R. Marzouki, Corrosion Inhibition of N80 Steel by Newly Synthesized Imidazoline Based Ionic Liquid in 15% HCl Medium: Experimental and Theoretical Investigations, International Journal of Electrochemical Science, 2021, 16(11), 211139.
S.M. Shareef, Power quality enhancement by optimally placing the UPQC in the distribution system: A hybrid optimization model, Journal of Computational Mechanics, Power System and Control, 2021, 4(1), 26-34.
M. Bouayed, H. Rabaa, A. Srhiri, J.Y. Saillard, A.B. Bachir, A. Le Beuze, Experimental and theoretical study of organic corrosion inhibitors on iron in acidic medium, Corrosion Science, 1999, 41(3), 501-517.
G. Gece, S. Bilgiç, Quantum chemical study of some cyclic nitrogen compounds as corrosion inhibitors of steel in NaCl media, Corrosion Science, 2009, 51(8), 1876-1878.
R.K. Roy, S. Pal, K. Hirao, On non-negativity of Fukui function indices, The Journal of chemical physics, 1999, 110(17), 8236-8245.
R.R. Contreras, P. Fuentealba, M. Galván, P. Pérez, A direct evaluation of regional Fukui functions in molecules, Chemical Physics Letters, 1999, 304(5-6), 405-413.
U. Umaru, A.M. Ayuba, Quantum chemical calculations and molecular dynamic simulation studies on the corrosion inhibition of aluminium metal by myricetin derivatives, Journal of New Technology and Materials, 2020, 10(02), 18-28.
DOI: http://dx.doi.org/10.13171/mjc02402281774nyijime
Refbacks
- There are currently no refbacks.
Copyright (c) 2024 Mediterranean Journal of Chemistry
