A study of docking and dereplication of extracts from Tradescantia pallida and their in vitro cytotoxic activities to control dengue mosquitoes
Abstract
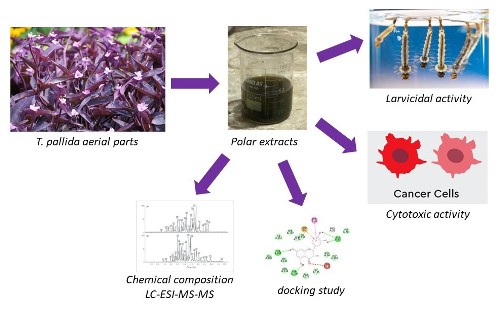
Tradescantia pallida is a plant known for its luxuriant purple leaves. This study aimed at extracting ethyl acetate extract (EAE) and ethanolic extract (EE) from T. pallida aerial parts to identify their phenolic compounds by liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI-MS-MS) using commercial patterns and at evaluating their larvicidal and cytotoxic activities against Aedes aegypti larvae and tumor cell lines, respectively. To assess their larvicidal activity, different concentrations of EAE and EE (10, 100, and 1000 μg/mL) were prepared with sterile distilled water and dimethyl sulfoxide (DMSO) (1%). Then, 20 mL of every solution and 25 third-stage larvae were placed in plastic cups. Cytotoxic activity of extracts was evaluated at concentrations ranging from 31.25 to 1000 µg/mL in tumor and non-tumor human cell lines. EAE and EE were found to be toxic to A. aegypti larvae since LC50 values were 435.0 and 480.5 µg/mL, respectively. Extracts showed no cytotoxic activity after the 24-hour treatment at 1000 µg/mL concentrations. LC-ESI-MS-MS results revealed that quercetin is the compound found at the highest concentration in both ethanol and ethyl acetate extracts; it highlights its significant role in both extraction methods and its prominence in the docking study. The docking analysis of quercetin against the crystal structure of arylalkylamine N-acetyltransferase 2 (4FD5) from A. aegypti yielded a binding energy of -6.7 kcal/mol. Additionally, details about the acute toxicity profile of quercetin and its possible toxicity targets have been provided. Results showed that extracts under evaluation exhibited larvicidal effects and showed no cytotoxicity to human cells.
Full Text:
PDFReferences
Souza SJP, Guaraldo AC, Honório NA, Câmara DCP, Sukow NM, Machado ST, Santos CND, Costa-Ribeiro MCV. Spatial and temporal distribution of Aedes aegypti and Aedes albopictus oviposition on the coast of Paraná, Brazil, a recent area of Dengue virus transmission. Trop Med Infect Dis. 2022, 7, 246.
Bezerra JMT, Sousa SC, Tauil PL, Carneiro M, Barbosa DS. Entry of dengue virus serotypes and their geographic distribution in Brazilian federative units: a systematic review. Rev Bras Epidemiol. 2021, 24, E210020.
Moura PF, Betim FCM, Oliveira CF, Dias JFG, Montrucchio DP, Miguel OG, Auer CG, Miguel MD. Atividade larvicida de extractos de Diplodia pinea frente à Aedes aegypti. Res Soc Develop. 2021, 10, e6710212295.
Manzano P, García OV, Malusín J, Villamar J, Quijano M, Viteri R, Barragán A, Orellana-Manzano A. Larvicidal activity of ethanolic extract of Azadirachta indica against Aedes aegypti larvae. Rev Fac Nac Agron. 2020, 73, 9315-9320.
Mituiassu LMP, Serdeiro MT, Vieira RRBT, Oliveira LS, Malek M. Momordica charantia L. extracts Against Aedes aegypti larvae. Braz J Biol. 2022, 82, e236498.
Silva AMAP, Silva AM, Masson R, Mota RD, Costa NC, Ribeiro EE, Loureiro WAS, Figueiredo PMS. Avaliação da atividade antimicrobiana da planta Tradescantia pallida Munt (taboquinha roxa). Rev Bras Pl Med. 2015, 17, 374-378.
Carvalho RM, Machado JLC, Aguiar RPS, Mata AMOF, Silva RR, Teixeira JS, Alencar MVOB, Islam MT, Melo-Cavalcante AAC. Tradescantia pallida as a biomonitoring tool to assess the influence of vehicle exhaustion and benzene derivatives. Afr J Biotechnol. 2017, 16, 280-287.
Menegazzo RF, Bortolucci WC, Oliveira HLM, Menegazzo AW, Gonçalves JE, Fernandez CMM, Gazim ZC, Lopes AD. Chemical composition of Tradescantia pallida (Rose) D.R. Hunt var. purpurea Boom (Commelinaceae) essential oil. Nat Prod Res. 2022, 36, 396-400.
Cabral FV, Fernandes CC, Dias ALB, Ribeiro AB, Squarisi IS, Tavares DC, Crotti AEM, Moreira FF, Miranda MLD. Hexane extract from Tradescantia pallida (Rose) D.R. Hunt (Commelinaceae): its volatile constituents and in vitro antifungal and cytotoxic activities. Braz Arch Biol Technol. 2022, 65, e20210621.
Abubakar AR, Haque M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J Pharm Bioall Sci. 2020, 12, 1-10
Betim FCM, Oliveira CF, Souza AM, Szabo EM, Zanin SMW, Miguel OG, Miguel MD, Dias JFG. Ocotea nutans (Nees) Mez (Lauraceae): chemical composition, antioxidant capacity and biological properties of essential oil. Braz J Pharm Sci. 2019, 55, e18284.
Carnizello AP, Barbosa MIF, Martins M, Ferreira NH, Oliveira PF, Magalhães GM, Batista AA, Tavares DC. In vitro and in vivo antitumor activity of a novel carbonyl ruthenium compound, the ct-[RuCl(CO)(dppb) (bipy)] PF-6[dppb=1,4-bis(diphenylphosphine)butane and bipy=2,2’-bipyridine]. J Inorg Biochem. 2016, 164, 42-48.
Trott O, Olson AJ. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comp Chem. 2010, 31, 455–461.
Biovia DS. 2015. Discovery studio modeling environment. San Diego, CA: Dassault Systemes.
Banerjee P, Eckert AO, Schrey AK, Preissner R. 2018. ProTox-II: a webserver for the prediction of toxicity of chemicals. Nucleic Acids Research, 46(W1), W257–W263.
Rolta R, Salaria D, Sharma PP, Sharma B, Kumar V, Rathi B, Verma M, Sourirajan A, Baumler DJ, Dev K. Phytocompounds of Rheum emodi, Thymus serpyllum, and Artemisia annua Inhibit Spike Protein of SARS-CoV-2 Binding to ACE2 Receptor: In Silico Approach. Curr Pharmacol Rep. 2021, 7, 135–149.
Rolta R, Yadav R, Salaria D, Trivedi S, Imran M, Sourirajan A, Baumler DJ, Dev K. (2021). In silico screening of hundred phytocompounds of ten medicinal plants as potential inhibitors of nucleocapsid phosphoprotein of COVID-19: an approach to prevent virus assembly. J Biomol Struct Dynamics, 2021, 39, 7017–7034.
Salaria D, Rolta R, Sharma N, Dev K, Sourirajan A, Kumar V. In silico and In vitro evaluation of the anti-inflammatory and antioxidant potential of Cymbopogon citratus from North-western Himalayas. BioRxiv, 2020, 05, 124982.
Imtiaz F, Islam M, Saeed H, Ahmed A, Agshar M, Saleem B, Farooq MA, Khan DH, Peltonen L. Novel phytoniosomes formulation of Tradescantia pallida leaves attenuates diabetes more effectively than pure extract. J Drug Deliv Sci Technol. 2023, 83, 104399.
Butnariu M et al. A Review on Tradescantia: Phytochemical Constituents, Biological Activities and Health-Promoting Effects. Front. Biosci. 2022, 27, 197.
Lima SR, Felisbino DG, Lima MRS, Chang R, Martins MM, Goulart LR, Andrade AA, Messias DN, Santos RR, Juliatti FC, Pilla V. Fluorescence quantum yield of natural dye extracted from Tradescantia pallida purpurea as a function of the seasons: preliminary bioapplication as a fungicide probe for necrotrophic fungi. J Photochem Photobio B: Biol. 2019, 200, 111631.
Kamiya M, Mori T, Nomura M, Inagaki T, Nonogaki T, Nagatsu A, Yamagishi Y, Mikamo H, Ikeda Y. Tradescantia pallida extract inhibits biofilm formation in Pseudomonas aeruginosa. Nagoya J Med Sci. 2019, 81, 439-452.
Li CY, Zhang ZC, Mao JY, Shi LF, Zheng Y, Quan JL. Preparation of Tradescantia pallida-mediated zinc oxide nanoparticles and their activity against cervical cancer cell lines. Trop J Pharm Res. 2017, 16, 494-500.
Kim JS, Kim KC. Antioxidant and α-glucosidase inhibitory activities of Tradescantia pallida (Rose) Hunt leaf extract and fractions. Korean J Medicinal Crop Sci. 2016, 24, 222-227.
Rocha AN, Carvalho EM, Mauad JRC, Mussury RM. Tradescantia pallida L. (Commelinaceae) influences the activity of oviposition and feeding of Plutella xylostella (Lepidoptera: Plutellidae). Res Soc Develop. 2021, 10, e57610817583.
Shi Z, Lin M; Francis FJ. Anthocyanins of Tradescantia pallida. Potential food colorants. J Food Sci. 1992, 57, 761-765.
Silva LS, Martins CF, Abrão FY, Romano CA, Bezerra SF, Borges LL, Santos AH, Cunha LC, Neto JRO, Fiuza TS, Paula JR. Comparative study of the chemical composition, larvicidal, antimicrobial and cytotoxic activities of volatile oils from E. punicifolia leaves from Minas Gerais and Goiás. Res Soc Develop. 2021, 10, e34101119354.
Ghosh P, Dutta A, Biswas M, Biswas S, Hazra L, Nag SK, Sil S, Chatterjee S. Phytomorphological, chemical and pharmacological discussion about Commelina benghalensis Linn. (Commelinaceae): a review. Pham Innov J. 2019, 8, 12-18.
Calderón AI, Romero LI, Ortega-Barría E, Brun R, Correa AMD, Gupta MP. Evaluation of larvicidal and in vitro antiparasitic activities of plants in a biodiversity plot in the Altos de Campana National Park, Panama. Pharm Biol. 2006, 44, 487-498.
Zuharah WF, Yousaf A, Ooi KL, Salaiman SF. Larvicidal activities of family Anacardiaceae on Aedes mosquitoes (Diptera: Culicidae) and identification of phenolic compounds. J Kind Saud Univer Sci. 2021, 33, 101471.
Pessoa LZS, Duarte JL, Ferreira RMA, Oliveira AEMFM, Cruz RAS, Faustino SMM, Carvalho JCT, Fernandes CP, Souto RNP, Araújo RS. Nanosuspension of quercetin: preparation, characterization and effects against Aedes aegypti larvae. Braz J Pharmacog. 2018, 28, 618-625.
Hartmann I, Silva A, Walter ME, Jeremias WJ. Investigação do efeito larvicida de Petiveria alliacea (Guiné) sobre as larvas de mosquitos da espécie Aedes aegypti. Rev Virtual Quim. 2018, 10, 529-541.
Catelan TBS et al. Evaluation of Toxicity of Phenolic Compounds Using Aedes aegypti (Diptera: Culicidae) and Artemia salina. Adv Infect Dis. 2015, 5, 48-56.
DOI: http://dx.doi.org/10.13171/mjc02409141798miranda
Refbacks
- There are currently no refbacks.
Copyright (c) 2024 Mediterranean Journal of Chemistry
