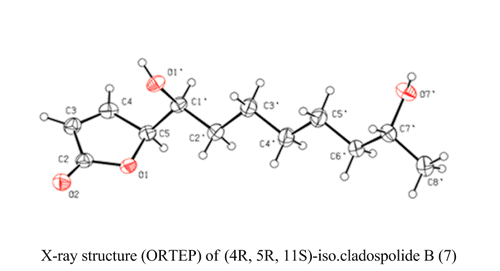
First total synthesis of (4R,5R,11S) and (4R,5R,11R)-iso-cladospolide B
Abstract
The two enantiomers of natural products (4S, 5S, 11R)-and (4S, 5S, 11S)-iso-cladospolide B have been synthesized and their structures unambiguously confirmed by X-ray crystallographic analysis. Key steps of the synthesis include the use of tri-O-acetyl-D-glucal as precursor for a chiral furan diol which, after side chain transformation, underwent singlet oxygen oxidation to afford the target butenolides
Full Text:
PDFDOI: http://dx.doi.org/10.13171/mjc.4.1.2015.17.02.10.25/yagamare
Refbacks
- There are currently no refbacks.
Copyright (c) 2015 Mediterranean Journal of Chemistry
