Equilibrium, kinetics and thermodynamics studies of textile dyes adsorption on modified Tunisian clay
Abstract
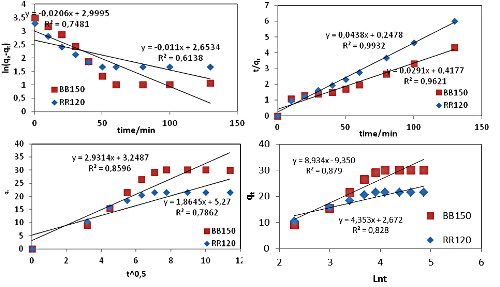
The adsorption capacity of two anionic textile dyes (RR120 and BB150) on DMSO intercalated Tunisian raw clay was investigated with respect to contact time, initial dye concentration, pH and Temperature. The equilibrium data were fitted into Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms. The kinetic parameters were calculated using pseudo-first order, pseudo second-order, intra-particle diffusion and Elovich kinetic models. The thermodynamic parameters (DH°, DS° and DG°) of the adsorption process were also evaluated.
Full Text:
PDFReferences
- UNEP/MAP/CAR/PP, 2002. “Prevention of the Pollution in the Textile Industry in the Mediterranean Regionâ€. CAR/PP document, Barcelona. Available online at: http://www.cprac.org/.
- A. Berez, F. Ayari, N. Abidi , G.Schafer and M. Trabelsi- ayadi, Adsorption-desorption processes of azo dye on natural bentonite: batch experiments and modeling, Clay Minerals, 2014, 49, (5), 747-763.
- F. Ayari, A. Berez, M. T. Ayadi, Biosorption of an anionic textile dye from aqueous solution by natural nano-adsorbant as solution to reduce toxic dye pollutant from wastewater Modelling and Kinetic study, International Journal of Engineering and Innovative Technology, 2014, 4 (6), 215-224.
- N. Abidi, E. Errais, J. Duplay, A. Berez, A. Jrad, G. Schafer, M. Ghazi, K. Semhi, M. T. Ayadi, Treatment of dye-containing effluent by natural clay, Journal of Cleaner Production, 2015, 86, 432-440.
- Y. Yang, C. Ladisch and M. R. Ladisch, Cellulosic adsorbents for treating textile mill effluents, Enzyme and Microbial Technology, 1988, 10 (10), 632-636.
- S. Rengaraj, S. H. Moon, Kinetics of adsorption of Co(II) removal from water and wastewater by ion exchange resins, Water Research, 2002, 36, 1783-1793.
- A.Y. Zahrima, N. Hilalb, Treatment of highly concentrated dye solution by coagulation/flocculation–sand filtration and nanofiltration, Water Resources and Industry, 2013, 3, 23–34.
- M.M. Nassar, M.S. El-Geundi, comparative cost of colour removel from textile effluents using natural adsorbents, Journal of Chemical Technology and Biotechnology, 1999, 50, 257-264.
- N. Naghmouchi and K. Nahdi, Adsorption of textile dyes on raw Tunisian clay: Equilibrium, kinetics and thermodynamics, Journal of Advances in Chemistry, 2015, 11 (6), 3685-3697.
- R. A. R. Da Silva and D. J. L. Guerra, Use of natural and modified kaolinite/ilite as adsorbent for removal methylene blue dye from aqueous solution, Journal of the Chilean Chemical Society, 2013, 58 (1), 1517-1519.
- I. Langmuir, “the constitution and fundamental properties of solids and liquids. Part I. Solids,†Journal of the American Chemical Society, vol.38, pp.2221-2295, 1916.
- I. Langmuir, the adsorption of gases on plane surfaces of glass, mica and platinum, Journal of the American Chemical Society, 1918, 40, 1361-1403.
- H.M.F. Freundlich, over the adsorption in solution, The Journal of Physical Chemistry, 1906, 57, 385-471
- H. M. F. Freundlich, Colloid and Capillary Chemistry, Methuen and Co. Ltd, London, UK, 1926.
- M. I. Temkin, adsorption equilibrium and kinetics of processes on non homogeneous and on the interactions between the adsorbed molecules, The Journal of Physical Chemistry, 1941, 15, 296-332.
- M.I. Tempkin, V. Pyzhev, Kinetics of ammonia synthesis on promoted iron catalyst, Acta Phys. Chim. (USSR), 1940, 12, 327–356.
- C. Aharoni, M. Ungarish, Kinetics of activated chemisorption. Part 2.-Theoretical models, Journal of the Chemical Society Faraday Transactions, 1977, 73, 456–464.
- M.M. Dubinin, the potential theory of adsorption of gases and vapors for adsorbents with energetically non-uniform surface, Chemical Reviews, 1960, 60, 235–266.
- A. Dabrowski, Adsorption-from theory to practice, Advances in Colloid and Interface Science, 2001, 93, 135-224.
- A. Gunay, E. Arslankaya, I. Tosun, Lead removal from aqueous solution by natural and pretreated clinoptilolite: adsorption equilibrium and kinetics, Journal of Hazardous Materials, 2007, 146, 362–371.
- M. Horsfall, A. I. spiff, A. A. Abia, Studies on the influence of mercaptoacetic acid (MAA) modification of cassava (manihot sculenta cranz) waste biomass on the adsorption of Cu2+ and Cd2+ from aqueous solution, Korean Chemical Society, 2004, 25, 969-976.
- R. Cavet, Le sol - Propriétés et fonction; Tome 1 : Edition France Agricole, 2003.
- S. Lagergren, Zur Theorie der Sogenannten Adsorption Gelöster Stoffe, Kungliga Svenska Vetenskapsakademiens, Handlingar, 1898, 24, 4, 1-39.
- Y. S. Ho, G. McKay, Kinetic models for the sorption of dye from aqueous solution by wood, Process Safety and Environmental Protection, 1998, 76, 183-191.
- J. R. Weber, J.C. Morris, Kinetics of adsorption on carbon from solution, Journal of the Sanitary Engineering Division, 1963, 89, 31–59.
- D. Yue, Y. Jing, J. Ma, C. Xia, X. Y., Y. Jia, Removal of neutral red from aqueous solution by using modified hectorite, Desalination, 2011, 267 , 9–15.
- S. J. Elovich, J. H. Schulman (Ed.), Proceedings of the Second International Congress on Surface Activity, Academic Press, Inc., New York, 1959, vol.11, p. 253.
- S. H. Chien, W. R. Clayton, Application of Elovich equation to the kinetics of phosphate release and sorption on soils, Soil Science Society of America Journal, 1980, 44, 265-268.
- K. S. Shahraki, T. Ghosh, K. Mahajan, A. J. Ajji, P. Carreau, Effect of dry grinding on chemically modified kaolin, Applied Clay Science, 2015, 105–106, 100–106.
- D. L. Sparks, Kinetics of Soil Chemical Processes, Academic Press, New York, 1989.
- G. Sposito, Surface reaction in natural colloidal system, Chimia, 1989, 43, 169-176.
- G. Rytwo, E. R. Hitzky, Enthalpies of adsorption of methylene blue and crystal violet to montmorillonite, Journal of Thermal Analysis and Calorimetry, 2003, 71, 751-759.
- Z. Bouberka, S. Kacha, M. Kameche, S. Elmaleh, Z. Derriche, Sorption study of an acid dye from an aqueous solutions using modified clays, Journal of Hazardous Materials, 2005, 119, 117-124.
Refbacks
- There are currently no refbacks.
Copyright (c) 2016 Mediterranean Journal of Chemistry
