Novel pyrano[3,2-c]chromene derivatives via a green one-pot three component: Synthesis, characterization , antioxidant, antibacterial and anti-inflammatory activities
Abstract
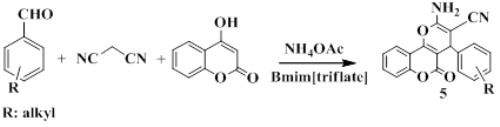
A simple, green, efficient and economical procedure for the synthesis of , pyrano[3,2-c]chromene derivatives by using a one-pot three-component condensation of 4-Hydroxycoumarin, various arylaldehydes and malonitrile in 1-butyl-3-methylimidazolium triflate as green media is described. This reaction proceeded under mild conditions with the use of an inexpensive and readily available catalyst, high to excellent yields, and simple workup procedure. In addition; the compounds 5 were investigated for anti-oxidant activities by super oxide radical; DPPH (2,2-Diphenyl-1-picrylhydrazyl); and hydroxyl radical scavenging assays; in which most of them displayed significant antioxidant activities. Furthermore; compounds 5  were evaluated for anti-inflammatory activity by indirect haemolytic and lipoxygenase inhibition assays and revealed good activity. Most of the new compounds 5 exhibited moderate antibacterial activity.Â
Full Text:
PDFReferences
I.Ugi, A. Domling, W. Horl, Endeavor., 1994, 18, 115.
C.Hulme, V. Gore, Med. Chem.,2003, 1, 51.
K. S Nirav, P. P. Manish, G. P. Ranjan, Phosphorus, Sulfur Silicon Relat. Elem., 2009, 184, 2704-2719.
H. Naeimi, Z. Rashid, A.H. Zarnani, R. Ghahremanzadeh, J.Chem., 2013, Article ID 169695, 9.
E. Rajanarendar, P. Venkateshwarlu, S.R. Krishna, K. G . Reddy, K. Thirupathaiah, Green and Sustainable Chemistry., 2015, 5, 107-114.
K. Kumaravel and G. Vasuki, †Green Chemistry., 2009,vol. 11, no. 12, pp. 1945-1947.
S. L. Jain, S. Singhal and B. Sain, Green Chemistry., 2007,vol. 9, no. 7, pp. 740-741.
J. Wang, S. Daqing, Q. Zhuang, W. Xiangshan, Hu. Hongwen, Journal of Chemical Research. 2004, 12, 818 -820.
S. Rupam, S. Manas, M. Lekhok, C. Kushal, D.Prajapati, Synlett., 2010, 19 , 2847 - 2852 .
M. P. Goncharenko, Y. Sharanin, Russian Journal of Organic Chemistry. 1993, 29, 1218
L.E. Kaïm, L. Grimaud, J. Oble, Angew. Chem. Int. Ed., 2005, 44, 48, 7961.
M. Sinha, K. Khoury, E. Herdtweck, A. Dömling, Chem. Eur. J., 2013, 19, 25, 8048-8052.
M. Sinha, K. Khoury, E. Herdtweck, A. Dömling, Org. Biomol. Chem., 2013, 11, 29, 4792-4796.
Y.M.D. Chuanga, Y.S.Wanga, Y.Y. Kuob, H. P. Tsaia, W.L. Shyura, 2004. J. Ethnopharmacol., 95, 409-419.
B. Halliwell, J.M. C.Gutteridge, O. L. Arnoma, Anal. Biochem., 1987, 165, 215-219.
M. N. Nishimiki, K. Yagi, Biochem. Biophys. Commun., 1972, 46, 849-854.
U .A.Shinde, K. R.Kulkarni, A. S. Phadke, A. M. Nair, D. V. J. Mungantiwar, M. N. Saraf, Ind. J. Exp. Biol., 1999, 371, 258-261.
H. G.Boman, U. Kaletta, Biochem. Biophys. Acta., 1957, 24, 619- 623.
K. H. Ahn, H. Kim, J. Kim, R. Jeomg, S. C. Kang, T. S. H. T. Shin, G. Lim, J. Bull. Korean Chem. Soc., 2002, 23, 626-628.
R. Hagiwara, Y. Ito, Journal of Fluorine Chemistry., 2000. 105, 2, 221-227.
T. Welton, Chemical Reviews., 1999, 99, 8, 2071-2083.
M. Earle, J. Seddon, Pure and Applied Chemistry., 2000, 72, 7, 1391-1398.
H. Olivier, Journal of Molecular Catalysis A., 1999, 146, 1-2, 285-289.
J .S. Yadav, B. Reddy, P. Sreedhar, Journal of Molecular Catalysis A., 2007, 270, 160-163.
R. Sheldon, Chemical Communications., 2001, 23, 2399-2407.
P.A.Wayne, National Committee for clinical laboratory standard. Referece method for broth dilution antifungal susceptibility testing of conidium - forming filamentous fungi. Proposed standard M38-P. 1998. National Committee for clinical laboratory standard.
Y.S.Shim, K.C.Kim, D.Y.Chi, K.H.Lee, H. Cho, Bioorg. Med. Chem. Lett, 2003, 13, 2561-2563.
Refbacks
- There are currently no refbacks.
Copyright (c) 2016 Mediterranean Journal of Chemistry
