Tricalcium phosphate powder: Preparation, characterization and compaction abilities
Abstract
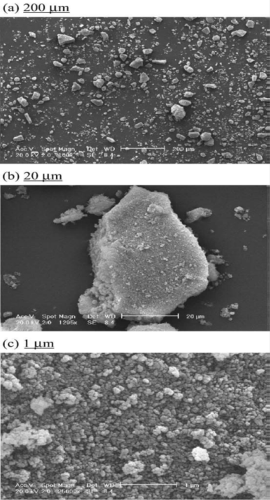
In this work, we characterize tricalcium phosphate powders Ca9(HPO4)(PO4)5(OH) resulting from a reaction between calcium hydroxide and orthophosphoric acid at room temperature, without pH adjustment and in absence of ionic impurities. The prepared powder has an atomic ratio Ca/P of 1.512 ± 0.005. The real density is 2.68 ± 0.02 g/cm3 and the specific surface area is 80 ± 02 m2 /g. During compression, the microstructure of Cadeficient apatite powder with the presence of HPO4 groups seems to support the cohesion between particles. The transmission ratio is 90%, the transfer ratio is 41.8 and the ratio of the die-wall friction is 0.22. These results show that apatitic tricalcium powder gives a good aptitude to the compaction which leads to a good tensile strength (0.79 MPa). The heat treatment of the prepared powder shows the precise temperature for the formation of pyrophosphate, β-TCP and α-TCPa phases. The purity and aptitude to compaction of the prepared powders are very promising for pharmaceutical and medical applications.
Full Text:
PDFReferences
R. Z. LeGeros, S. Lin, R. Rohanizadeh, D. Mijares, J. P. LeGeros, Journal of Materials Science: Materials in Medicine, 2003, 14(3), 201-209.
M.H. Prado Da Silva, A.F. Lemos, J.M.F. Ferreira, J.D. Santos, J. Non-Cryst. Sol. 2002, 304, 286.
J.S.V. Albuquerquel, J.V. Ferreira Neto, J.I.L. Almeida Jr., D.O. Lima, R.E.F.Q. Nogueira, M.H. Prado da Silva, J Key Engrg. Mater, 2003, 240, 23.
L. Galois, D. Mainard, J.P. Delagoutte, International Orthopaedics (SICOT), 2002, 26, 109-115.
T. Von Arx, D. L. Cochran, J. S. Hermann, R. K. Schenk, D. Buser, J Clinical Oral Implants Research, 2001, 12(3), 260-269.
B. Mirhadi , B. Mehdikhani, N. Askari, J Processing and Application of Ceramics ,2011,5 (4), 193-198.
A. Massit, A. Yacoubi, B. Chafik El Idrissi, K. Yamni, Verres, Céramiques & Composites, 2015, 4(1), 1-6.
M. Akao, H. Aoki, K. Kato, A. Sato, J. Mater. Sci., 1982,17, 243-46 .
AFNOR NF S 94-066.
A. Michrafy, M.S. Kadiri, J.A. Dodds, J Chem. Eng. Res. Des. 2003, 81, 946-952.
N. Sarath Chandra Reddy , D.M. Dewaikar, G. Mohapatra, International Journal of Advanced Civil Engineering and Architecture Research, 2013, 2(1), 32-41.
A. Mortier, J. Lemaétre, P.G. Rouxhet. J Thermochim.Acta, 1989, 143, 265-282.
A. Destainville, E. Champion, D. Bernache-Assolant, E. Labore.J Matter. Chem.Phys. 2003, 80, 269-277.
H.S. Ryu, H.J. Youn, K.S. Hong, B.S. Chang, C.K. Lee, S.S. Chung, An improvement in, Biomaterials, 2002, 23, 909-914.
DOI: http://dx.doi.org/10.13171/mjc63/01702031740-abida
Refbacks
- There are currently no refbacks.
Copyright (c) 2017 Mediterranean Journal of Chemistry
