Theoretical study of the interaction between vanadium oxide and HY-Zeolite elucidating loss activity of the catalyst
Abstract
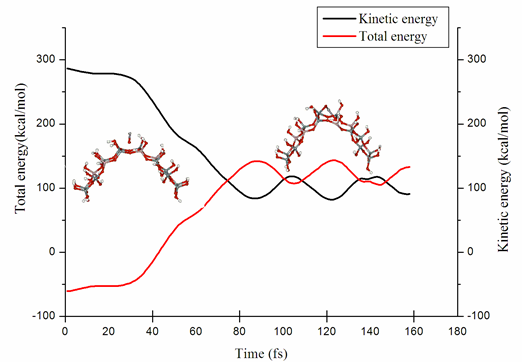
The study of the interaction between vanadium oxide and the HY-zeolite using molecular dynamics interaction was carried out for two systems: i) when vanadium oxide penetrates a zeolite ring at its center, and ii) when vanadium oxide impacts the zeolite surface model. The dynamical effects are used to investigate the reduced activity and eventual degradation of the catalyst for the vanadium oxide presence. In the first case, we observe the breaking of an OH-bond that belongs to the acid site. This is related to an initial loss activity stage of the catalyst. In the second case, vanadium oxide is weakly adsorbed onto the zeolite surface velocity depending. Density functional theory, with nonlocal exchange and correlation functional and the basis set of double numerical accuracy, is used to analyze the electronic structure. It was used in combination with Born-Oppenheimer dynamics to perform calculations.
Full Text:
PDFReferences
- R.H. Harding, A.W. Peters, J.R.D. Nee New developments in FCC catalyst technology. Appl Catal A: General. 2001, Dec; 221(1), 389-396.
- P.I. Premovic, D.M. Dordevic, M.S. Pavlovic. Vanadium of petroleum asphaltens and source kerogens. Fuel. 2002, Oct; 81(15), 2009-2016.
- J. Haber, M. Witko, and R. Tokarz. Vanadium pentoxide I. Structures and properties. Appl Catal A General. 1997, Sept; 157(1), 3-22.
- R. Dimitrova, Y. Neinska, M. Mihályi, G. Pal-Borbély, M. Spassova. Reductive solid-state ion exchange as a way to vanadium introduction in BZSM and BBeta zeolites. App Catal A: General. 2004, Mar; 266(1), 123-127.
- L.A. Pine. Vanadium-catalyzed desrtruction of USY zeolite. J. Catal. 1990, Sept; 125(2), 514-524.
- M. Xu, X. Liu, R.J. Madon. Pathway for Y-zeolite destruction: the rol of sodium and vanadium. J Catal. 2002, Apr; 207(2), 237-246.
- H.S. Cerqueira, G. Caeirob, L. Costac, F.R. Ribeiroc, Deactivation of FCC catalysts. J. Mol Catal A, Chemical. 2008 Sept; 292(1), 1-13.
- U.J. Etim, B. Xu, R. Ullah, Z. Yan. Effect of vanadium contamination on the framework and micropore structure of ultra-stable Y-zeolite. J. Colloid Interface Sci., 2016, Oct; 463,188-198.
- J.B. Parise, D.R. Corbin, L. Abrams, D.E. Cox. Structure of dealuminated lindeY-zeolite. Acta Cryst. 1984, May; C40(1), 1493-1497.
- M. Niwa, K. Susuki, K. Isamoto, N. Katada. Identification and measurement of strong bronsted acid side in ultrastable Y-zeolite. J. Phys Chem B. 2006, Oct; 110, 264-269.
- P.J. Hay, A.Y. Redondo Guo. Theoretical studies of pentene cracking on zeolite: c-c β-scission process. Catal Today. 1999;50, 517-523.
- I.P. Zaragoza, L.A. Garcia-Serrano, R. Santamaria. Selectivity of a model zeolite ring over hydrocarbons with different symmetry travelling with different orientations and speeds. J. Phys Chem B. 2005, Dec; 109,705.
- R.G. Parr, W. Yang. Density functional theory of atoms and molecules. Oxford University Press: New York 1989. 333 p.
- S.M. Valiev, E.J. Bylaska, N. Govind, K. Kowalski, T.P. Straatsma, H.J.J. Van Dam, D. Wang, D.J. Nieplocha, E. Apra, T.L. Windus, W.A. de Jong. N.W.Chem: A comprehensive and scalable open-source solution for large scale molecular simulations. Comput Phys Commun. 2010, Sept: 181(9), 1477-1489.
- A.D. Becke. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A. 1988 Sept; 38, 3098.
- C. Lee, W. Yang, R.G. Parr. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B. 1988, Jan; 37, 785.
- A.RamÃrez-Solís . On the performance of local, semilocal, and nonlocal exchange-correlation functionals on transition metal molecules. J. Chem Phys. 2007, Jun; 126(22),224105.
- P.J. Hasnip, K. Refson, M.I.J. Probert, J.R. Yates, S.J. Clark, C.J. Pickard. Density functional theory in the solid state. Phil Trans R Soc A. 2014, Feb; 372,1-26.
I.P. Zaragoza, J.H. Pacheco-Sánchez, I. Echevarria-Chan, A. Bravo-Ortega. DFT study of interaction between a hydrogen molecule and AgY-zeolite. Rev Mex Fis. 2014, Nov; 60(6), 460-465.
DOI: http://dx.doi.org/10.13171/mjc62/01701082306-zaragoza
Refbacks
- There are currently no refbacks.
Copyright (c) 2017 Mediterranean Journal of Chemistry
