Investigation of the Self-Diffusion Coefficients of Trivalent Gd3+ in aqueous solutions: The Effect of Hydrolysis and nitrate ion association
Abstract
In recent work, we have deduced that berkelium and gadolinium, which have 4f and 5f with half-filled configurations, also display similar diffusion properties. Results, using self-diffusion coefficients to study thermodynamic properties, show very similar behavior, which is understood given their similarities in charge, ionic size, mobility, conductance, and hydration number. In this paper we undertook a study of Gd3+ as an aid for deducing the thermodynamics properties of the Bk3+ .
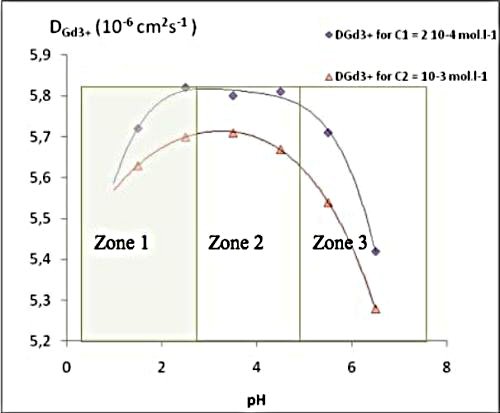
In first part we compare magnitudes of transport (self-diffusion coefficient) of the trivalent aquo ions of Gd III for two concentrations, C1 = 2 10-4 mol.L-1, C2 = 10-3 mol.L-1 , and in perchlorate and nitrate media . In second part, we studied the variation of D with the medium’s pH. Self-diffusion coefficients, D, of the trivalent f-elements aqua ions have been determined in an aqueous electrolyte support of Gd(NO3)3 and Nd(ClO4)3 and at a pH=2.5. (HNO3, HClO4 ) and at 25 °C using the open-end capillary method (O.E.C.M.).
The variation of D versus pH shows three zones
We changed the NO3- counter ion with ClO4- ,and found that measurements must be carried in perchloric acid medium so as to avoid pairing phenomena. Using association constants between Gd3+ and NO3- , and a hydration constant, we estimated self-diffusion coefficients for Gd(NO3)2+ and Gd(OH)2+ .
The results justified our choice of working at pH = 2.5 which constitute an optimum condition to avoid hydrolysis and pairing phenomena. Our results were confirmed by mobility measurements using an electro migration technique.
Full Text:
PDFReferences
- Y. Marcus , Chem. Rev,1988 ,88,1475-1498
- Z. Szab´o , T. Toraishi , V. Vallet , Ingmar Grenthe Coordination Chemistry Reviews, 2006, 250 , 784–815
- R. Besbes, N. Ouerfelli , A.Abdelmanef and H.Latrous,IOP Conf. Series: Materials Science and Engineering, 2010, 9, 012079
- H. Latrous, R. Besbes and N.Ouerfelli J. Mol. Liq , 2008 , 138 , 51–54
- H. Latrous , special publication- Royal Society of Chemistry, 2006 , 305, 290-292
- H. Latrous, J. Oliver, M. Chemla, Zeitchrift Phys. Chem. Neue Folge, Bd, 1988 ,159, 195 (S).
- H. Latrous, M. Ammar, J. M'halla, Radiochem. Radioanal. Lett, 1982, 53/1 ,33.
- H. Latrous, Rev. Fac. Sci. Tunis 1 (1981) 75, Chem. Abstr. 1981, 99 , 128643.
- N. Ouerfelli , H. Latrous, M. Ammar, J. Mol. Liq. 2009, 146 ,52–59.
- J. M'Halla, M. Chemla, R. Bury and F. David, J. Chim. Phys. 1988 , 85(1), 121.
- P. Lindqvist-Reis, R. Klenze, G. Schubert, T. Fanghänel, J. Phys. Chem., 2005, B 109 ,3077.
- J S. Anderson & K. Saddington, J.Chem.Soc., 1948 , 5 Suppl ,381.
- R. Mills & A.W.Godbole, Aust.J.Chem., 1958 , 11, 1.
- P. Turq, F. Lantelme & M. Chemla, Electrochim.Acta, 1969, 14 , 1081.
- J. M’Halla, P.Turq & M.Chemla, J.Ch.Soc.Far.Trans.I, 1987, 74, 2320.
- F. Kepak & J. Kriva, , J.Inorg.Nucl.Chem., 1971, 33 , 1741-48.
- J. M’Halla, M.Chemla, R. Bury & F. David, J.Chim.Phys., 1988 , 85 N1, 121-133.
- K.H. Schmidt, J. C. Sullivan, S.Gordon & R. C. Tompson, J. Inorg. Nucl.Chem.Let. 1978 ,14 , 429-434.
- P. Lindqvist-Reis, C. Walther, R. Klenze, A. Eichhofer, T. Fanghanel, J. Phys. Chem. B, 2006 , 110 , 5279.
- P.Lindqvist-Reis, C.Apostolidis, J. Rebizant, A. Morgenstern, R. Klenze, O. Walter, T. Fanghänel, R. G. Haire. Angew. Chem. Int., 2007 , 46 919-22.
- S. Skanthakumar, M.R.Antonio, R. E.Wilson, L. Soderholm, Inorg.Chem.,2007, 46 3485.
- L. Rao and T. Guoxin , Inorg. Chem , 2009 , 48(3) , 961-970.
- S. Andersson, H. Nitsche and B.Sadowe, Radio. Chem. Acta , 2006, 94 ,issue 1, 59-61.
- J. O. Liljenzin, M. Nisson and G. Skarnemark , Radio. Chem. Acta , 2006 , 94 , issue 8, 469-474.
- H. Latrous, J. Oliver & M. Chemla, J.Radiochem.Radional.Lett., 1982, 53N1, 81-88.
- N. Ouerfelli, H. Latrous & M. Ammar, Proc. of the Int’l.Conf. on Cond.Matter Phys.
& Appl., Bahrain. 1992 , 415-18. (Omega Scientific Oxon, U.K.1993).
- N. Ouerfelli, M. Ammar & H. Latrous, 1994 J.Chim.Phys., 91 1786-1795.
- (a) J. A. Rard & F. H. Spedding, Rare Earth Research Conference, Michigan Traverse City, 1974,1.
(b) F. H. Spedding & J. A. Rard, J.Phys.Chem., 1974, 78, 1435.
- (a) L. Onsager, Annals New York Acad.Sci., 1945 , 46, 241. (b) L. Onsager, & Fuoss R M, J.Phys.Chem., 1932 , 36, 2689.
- R. A. Robinson & R. H. Stokes, 1959, Electrolytes Solutions. Butterworths. London.
- F. David, B. Fourest, J. Duplessis, H. Latrous, J. Oliver, M. Chemla, J. M'halla, Inorganica Chimica Acta, 1984 , 94, Issues 1-3, 75-76
- H. Weingärtner, B. M. Braun & J. M. Schmoll, J.Phys.Chem., 1987, 91 , 979.
- H. S. Harned & C. A. Blake, J.Amer.Chem.Soc., 1951, 53 ,4255.
- A. Easteal, R. Mills & L. A. Woolf, J.Phys.Chem., 1989, 93 , 4968 & 7517.
- Van Der Maarel, J.R.C, de Bleijser, J.Chem.Phys.Lett., 1988, 91 251.
- J. O’M. Bockris & A. K. N. Reddy, Modern Electrochemistry, 1970 ,Vol 1, Plenum Press, New York.
- L.Sidney phillips, F. V. Hale and F. Lenard Silvester , Thermodynamic Tables for Nuclear Wate Isolation, 1988, 1 , 24 , LBL-22860
- K. Sur Sandip, J. Magn.Res, Serie B, 1996, 111 ,105-108 .
- J. Allen , J. Bucher, D. K Shuh, N. M. Edelstein and I. Craig , Inorg. Chem., 2000, 39(3), 595-601 (Berkeley , LBNL California).
- R. G. Haire and T. Fanghanel, Ange. Chem. Int. Ed. 2010, 49, 6343-6347
DOI: http://dx.doi.org/10.13171/mjc.1.6.2012.27.07.18
Refbacks
- There are currently no refbacks.
Copyright (c) 2015 Mediterranean Journal of Chemistry
