Optimization of activated carbon from residues of oregano using experimental design method
Abstract
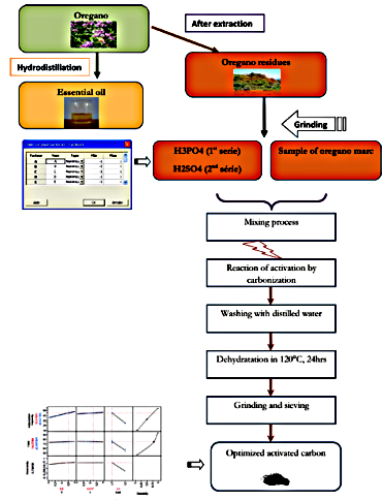
 A series of eight activated carbons were obtained by chemical activation of oregano marc, which is abundant waste in Morocco. The activated carbons responses (capacity of adsorption, and yield) were analyzed as a function of the experimental parameters (temperature, time and chemical agent), using the complete factorial design of 23 experiments. Each response has been described by a second order model that was adequate to predict responses in all experimental regions. The experimental values obtained were in good agreement with the values predicted from the models, with relatively small errors between the predicted and the actual values. The optimization of activated carbon, using the function of desirability, was carried out by JMP software. The optimal activated carbon is obtained when using 60 min as activation time, 350°C as activation temperature, and H3PO4 as chemical agent.
Full Text:
PDFReferences
- J. Novak , B. Christina, B. Langbehn, F. Pank, M. Skoula, Y. Gotsiou & C. M. Franz, Biochemical Systematics and Ecology, 2000, 28, 697–704.
- N. Aligiannis, E. Kalpoutzakis, S. Mitaku & I. B. Chinou, Journal of Agriculture Food Chemistry, 2001, 49, 4168– 4170.
- F. Sahin, M . Gulluce, D. Daferera, A. Sokmen, M . Sokmen, M. Polissiou, G. Agar, H. Ozer, Food Control, 2004, 15, 549–557.
- M. Kobya, E. Demirbas, E. Senturk and M. Ince, ‘Adsorption of Heavy Metal Ions from Aqueous Solutions by Actived Carbon Prepared from Apricot Stone’, Bioresource Technology, 2005, Vol. 96, N°13, 1518 - 1521.
- B. Harshavardhan, E. Stanley. Manahan and D.W. Larsen, An Actived Carbon Product Prepared from Milo (Sorghum Vulgare) Grain for Use in Hazardous Waste Gasification by Chem.Char Cocurrent Flow Gasification, Chemosphere, 1999, Vol. 39, N°1, 23 - 32.
- J. Avom, J.K. Mbadcam, M.R.L Matip and P. Germain, Adsorption Isotherme de l’Acide Acétique par des Charbons d’Origine Végétale, AJST, 2001, Vol 2, N°2, 1 - 7.
- F. Derbyshire, M. Jagtoyen, R. Andrews, A. Rao, I. MartinGullon and E. Grulke, ‘Carbon Materials in Environmental Applications’, In : Radovic, Editor, Chemistry and Physics of Carbon, New ork, Marcel Dekker, 2001, Vol. 27, N°1.
- J.Diaz-Teran, D.M.Nevskaia, J.L.G.Fierro, A.J.Lopez-Peinado, A.Jerez, Microporous and Mesoporous Materials, 2003, Vol. 60, 173-181.
- S. Behij, K. Djebali, H. Hammi, AH. Hamzaoui, A.M'nif, Optimisation of epsomite transformation into periclase using experimental design methodology, Journal of Chemometrics, 2011, 25, 59-66.
- R. Fezei, H. Hammi, A. M'nif, Optimization of sylvite transformation into arcanite using experimental design methodology, Journal of Chemometrics, 2008, 22, 122-129.
- M. Mercedes, Maroto-Valer, I. Dranca, T. Lupascu, R. Nastas, Effect of Adsorbate Polarity on Thermodesorption Profiles From Oxidized and Metal–Impregnated Activated Carbons, Elsevier, 2004, 2655-2659.
- M.G. Gronli, G. Varhegyi, C. Di Blasi, Thermogravimetric analysis and devolatilization kinetics of wood, Industrial and Engineering Chemistry Research, 2002, 41, 4201–4208.
- S. Munir, S.S. Daood, , W.Nimmo, A.M.Cunliffe, B.M. Gibbs, Thermal analysis and devolatilization kinetics of cotton stalk, sugar cane bagasse and shea meal under nitrogen and air atmospheres, Bioresources Technology, 2009, 100, 1413-1418.
- J.GOUPY, Introduction aux Plans d'expériences, Dunod, Paris, France, 2001.
- A.M. Puziy, O.I. Poddubnaya, A. Martinez-Alenso, F. Suarez-Garcia and J.M.D. Tascon, Synthetic Carbons Actived with Phosphoric Acid; Surface Chemistry and Ion Binding Properties, Carbon, 2002, 40, 1493 - 1505.
- F.K.H. Phoa, W.K. Wong, H.Xu, The need of considering the interactions in the analysis of screening designs, Journal of Chemometrics, 2009, 23, 545-553.
- J.GOUPY, " Etude comparative des plans d’expériences ", Revue de statistique appliquée, 1990, Tome 38 N°4, 5-44.
DOI: http://dx.doi.org/10.13171/mjc.1.5.2012.26.02.23
Refbacks
- There are currently no refbacks.
Copyright (c) 2015 Mediterranean Journal of Chemistry
