Synthesis of bicyclic oxepanes: an enantioselective approach to the western part of Shaagrockol C
Abstract
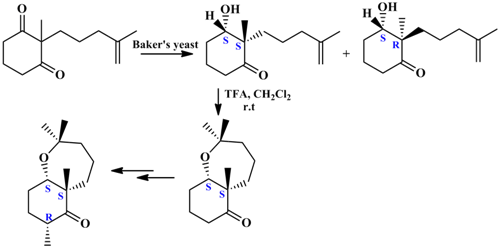
In this paper, we wish to report an efficient and flexible synthetic strategy towards the synthesis of Shaagrockol C. The strategy involves the construction of a bicyclic oxepane which possess several chiral centres. Such system is present in some complex natural products.
Full Text:
PDFReferences
- Isaacs, S.; Kashman, Y. Tetrahedron Lett., 1992, 33, 2227-2230.
- Isaacs, S.; Hizi, A.; Kashman, Y. Tetrahedron, 1993, 20, 4275-4282.
- Loya, S.; Tal, R.; Hizi, A. Journal of Natural Product, 1993, 56 (12), 2120-2125.
- Shumueli, U.; Carmely, S.; Groweiss, A.; Kashman,Y. Tetrahedron Lett., 1981, 22, 709-712.
- Ohtani, I.; Kusumi, T.; Kashman, Y.; Hakisawa, H. J. Org. Chem., 1991, 56, 1296-1298.
- Bokesch, H. R.; Stull, A. C.; Pannell, L. K.; Mc Kee, T. C.; Boyd, M. R. Tetrahedron Lett., 2002, 43, 3079-3081.
- Sullivan, B. W.; Faulkner, D. J.; Matsumoto, G. K.; Cun Heng He.; Clardy, J. J. Org. Chem., 1986, 51, 4568-4573.
- Fukami, A.; Ikeda, Y.; Kondo, S.; Naganawa, H.; Takeuchi, T.; Furuya, S.; Hirabayashi, Y. Shimoike, K.; Hosaka, S.; Watanabe, Y.; Umezawa, K. Tetrahedron Lett., 1997, 38, 1201-1202.
- Blackburn, C. L.; John Faulkner, D. Tetrahedron, 2000, 56, 8429-8432.
- Gray, C. A.; De Lira, S. P.; Silva, M.; Pimenta, E. F.; Thiemann, O. H.; Oliva, G.; Hajdu, E.; Andersen, R. J.; Berlinck, R. G. S. J. Org. Chem., 2006, 71, 8685-8690.
- Ziegler, F. E. Chem. Rev., 1988, 88, 1423-1452.
- Boyer, F. D.; Ducrot, P. H. Eur. J. Org. Chem., 1999, 1201-1212.
- Magnuson, S. R.; Laurenzino, L. S.; Rosen, N.; Danishefsky, S. J. J. Am. Chem. Soc., 1998, 120, 1615-1616.
- Ansell, M. F.; Thomas, D. A. J. Chem. Soc., 1961, 539-542.
- Garegg, R. J.; Regberg, T.; Stawinski, J.; Strömberg, R. J. Chem. Soc., Perkin Trans II. 1987, 3, 271-274.
- Starks, C. M. J. Am. Chem. Soc., 1971, 93, 195-199.
- Solodar, J. Tetrahedron. Lett., 1971, 12, 287-288.
- Docks, J. Synthesis, 1973, 441-456.
- Bram, G.; Galons, H.; Labidalle, S.; Loupy, A.; Miocque, M.; Petit, A.; Pigeon, P.; Sansoulet, J. Bull. Soc. Chim. Fr., 1989, 247-251.
- Yang, H. M.; Lin, C. L. J. Mol. Catal. A: Chem., 2003, 67-76.
- Butler, B.; Schultz, T.; Simpkins, N. S. Chem. Commun., 2006, 3634-3636.
- Carr, J. M.; Snowden, T. S. Tetrahedron, 2008, 64, 2897-2905.
- Elamparuthi, E.; Fellay, C.; Neuburger, M.; Gademann, K. Angew. Chem. Int. Ed. 2012, 51, 4071-4073.
- Czuk, R.; Glanzer, B. I. Chem. Rev., 1991, 91, 49-97.
- Servi, S. Synthesis, 1990, 1-25.
- Harrison, J. S.; Rose, A. H. (Eds). “The Yeastsâ€, 2nd Ed: Academic. Press: New York, 1987.
- Strathern, J. N.; Jones, E. W.; Broach, J. R. (Eds).“The Molecular Biology of the Yeast Saccharomyces ; Metabolism end Gene Expressionâ€. Cold Spring Harbor Laboratory Press: New York, 1983.
- Brooks, D. W.; Mazdiyasni, H.; Grothaus, P. G. J. Org. Chem., 1987, 52, 3223-3232.
- Brooks, D. W.; Mazdiyasni, H, Chakrabarti, S. Tetrahedron Lett., 1984, 25, 1241-1244.
- Grant. D. M.; Cheney, B. V. J. Am. Chem. Soc., 1967, 89, 5315-5318.
- Renouf, P.; Poirier, J. M.; Duhamel, P. J. Org. Chem., 1999, 64, 2513-2515.
- Dale, J. A.; Dull, D. L.; Mosher, H. S. J. Org. Chem., 1969, 34, 2543-2549.
- Hagiwara, H.; Uda, H. J. J. Chem. Soc., Perkin Trans I. 1991, 1803-1807.
- Krapcho, A. P. Synthesis, 1982, 805-822.
DOI: http://dx.doi.org/10.13171/mjc.2.3.2013.06.04.01
Refbacks
- There are currently no refbacks.
Copyright (c) 2015 Mediterranean Journal of Chemistry
