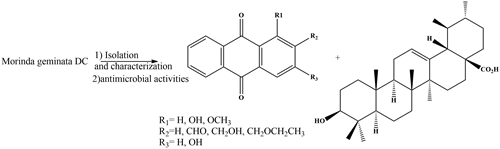
Antimicrobial anthraquinones and triterpenoid isolated from Morinda geminata DC (Rubiaceae)
Abstract
Full Text:
PDFReferences
- S. Ranasinghe, R. Ansumana, J. M. Lamin, A. S. Bockarie, U. Bangura, J. A.G. Buanie, D. A. Stenger, K. H. Jacobsen, Herbs and herbal combinations used to treat suspected malaria in Bo, Sierra Leone, J. Ethnopharmacol., 2015, 166, 200-204, DOI: 10.1016/j.jep.2015.03.028.
- K. Kamiya, New Anthraquinone and Iridoid from the Fruits of Morinda citrifolia, Chem. Pharm. Bull., 2005, 53(12), 1597-1599.
- B. Boolamou, A. Lapo, K. Camara, M. Assane, E. Bassene, and A. Samb, Activité anti-inflammatoire du décocté aqueux des écorces, de racines et de la tige de Morinda geminata; DC (Rubiaceae), Int. J. Biol. Chem. Sci., 2015, 8(4), 1871-1875, DOI:10.4314/ijbcs.v8i4.46.
- O. G. Nacoulma, Plantes médicinales pratiques médicinales du Burkina Faso: cas du plateau central. Tome II, 1996.
- A. Chevalier, Revue internationale de botanique appliquée et d'agriculture tropicale, Rev. Int. Bot. Appliquée Agric. Trop., 1947, 27 (299), 407-428.
- G. N. Njoroge and R. W. Bussmann, Diversity and utilization of antimalarial ethnophytotherapeutic remedies among the Kikuyus (Central Kenya), J. Ethnobiol. Ethnomedicine, 2006, 2(1), 8, DOI: 10.1186/1746-4269-2-8.
- P. Roland, Le Caractère magique originel des haies vives et de leurs constituants (Europe et Afrique occidentale), J. Agric. Trop. Bot. Appliquée, 1965, 12(6-8), 253-291.
- V. Basilevskaia, Plantes Médicinales de Guinée, Conakry, Imp. Patrice L., 1969, 270.
- J. Kerharo et J.-G. Adam, La pharmacopée sénégalaise traditionnelle: plantes médicinales et toxiques, Edition vigot et Frères, 1974, 1012, DOI: 10.2174/1385272003375923
- J. L. Pousset, Plantes médicinales africaines. Utilisation pratique. Agence de coopération culturelle et technique (ACCT), Paris, 1989, 156.
- L. Koroma and B. N. Ita, Phytochemical compounds and antimicrobial activity of three medicinal plants (Alchornea hirtella, Morinda geminata and Craterispermum laurinum) from Sierra, Afr. J. Biotechnol., 2009, 8 (22), 6397-6401.
- Y. Berger and A. Castonguay, The carbon-13 nuclear magnetic resonance spectra of anthraquinone, eight polyhydroxyanthraquinones and eight polymethoxyanthraquinones, Magn. Reson. Chem., 1978, 11(8), 375-377.
- Y. Berger, A. Castonguay, and P. Brassard, Carbon-13 nuclear magnetic resonance studies of anthraquinones Part II-hydroxymethoxyanthraquinones, acetoxymethoxyanthraquinones and naturally occuring anthraquinone analogues, Magn. Reson. Chem., 1980, 14(2), 103-108.
- M. Kitajima, U. Fischer, M. Nakamura, M. Ohsawa, M. Ueno, H. Takayama, M. Unger, J. Stockigt and N. Aim, Anthraquinones from ophiorrhiza pumila tissue and cell cultures, Phytochemistry, 1998, 48 (1), 107-111.
- K. Kamiya, W. Hamabe, S. Tokuyama, K. Hirano, T. Satake, Y. Kumamoto-Yonezawa, H. Yoshida, Y. Mizushina , Inhibitory effect of anthraquinones isolated from the Noni (Morinda citrifolia) root on animal A-, B- and Y-families of DNA polymerases and human cancer cell proliferation, Food Chem., 2010,118(3),725-730, DOI: 10.1016/j.foodchem.2009.05.053.
- P. Chang and K. H. Lee, Cytotoxic antileukemic anthraquinones from Morinda parvifolia, Phytochemistry, 1984, 23(8), 1733-1736.
- W. Xiang, Q. S. Song, H. J. Zhang, and S.P. Guo, Antimicrobial anthraquinones from Morinda angustifolia, Fitoterapia, 2008, 79(7), 501-504.
- A. A. Sittie, E. Lemmich, C. E. Olsen, L. Hviid, A. Kharazmi, F. K. Nkrumah, S. B. Christensen, Structure-activity studies: in vitro antileishmanial and antimalarial activities of anthraquinones from Morinda lucida, Planta Med., 1999, 65(3), 259-261.
- G. Rath, M. Ndonzao, and K. Hostettmann, Antifungal anthraquinones from Morinda lucida, Int. J. Pharmacogn., 1995, 33 (2), 107-114.
- C. Younos, A. Rolland, J. Fleurentin, M. C. Lanhers, R. Misslin, and F. Mortier, Analgesic and behavioural effects of Morinda citrifolia, Planta Med., 1990, 56(5), 430-434.
- K. Koumaglo, M. Gbeassor, O. Nikabu, C. De Souza, and W. Werner, Effects of three compounds extracted from Morinda lucida on Plasmodium falciparum, Planta Med., 1992, 58(6), 533-534.
- P. Chang and C. Chen, Isolation and characterization of antitumor anthraquinones from Morinda umbellata, Chin. Pharm. J. (Taipei), 1995, 47, 347-353.
- N. H. Ismail, A. M. Ali, N. Aimi, M. Kitajima, H. Takayama, and N. H. Lajis, Anthraquinones from Morinda elliptica, Phytochemistry, 1997, 45(8), 1723-1725.
- C. R. Faltynek, J. Schroeder, P. Mauvais, D. Miller, S. Wang, D. Murphy, R. Lehr, M. Kelley, A. Maycock, W. Michne, M. Miski, and A. L. Thunberg., Damnacanthal is a highly potent, selective inhibitor of p56lck tyrosine kinase activity, Biochemistry (Mosc.), 1995, 34(38), 12404-12410.
- T. Hiramatsu, M. Imoto, T. Koyano, and K. Umezawa, Induction of normal phenotypes in ras-transformed cells by damnacanthal from Morinda citrifolia, Cancer Lett., 1993, 73(2-3), 161-166.
- E. M. Palsson, M. Popoff, M. Thelestam, and L. A. O'Neill, Divergent roles for Ras and Rap in the activation of p38 mitogen-activated protein kinase by interleukin-1, J. Biol. Chem., 2000, 275 (11), 7818-7825.
- M. Kamata, R. p. Wu, D. S. An, J. P. Saxe, R. Damoiseaux, Mi. E. Phelps, J. Haung, and I. S. Y.Chen,
Cell-based chemical genetic screen identifies damnacanthal as an inhibitor of HIV-1 Vpr induced cell death, Biochem. Biophys. Res. Commun., 2006, 348(3), 1101-1106.
- F. L. Lin, J. L. Hsu, C. H. Chou, W. J. Wu, C. I. Chang, and H. J. Liu, Activation of p38 MAPK by damnacanthal mediates apoptosis in SKHep 1 cells through the DR5/TRAIL and TNFR1/TNF-α and p53 pathways, Eur. J. Pharmacol., 2011, 650(1), 120-129.
- L. N. Jasril, L. Y. Mooi, M. A. Abdullah, M. A. Sukari, and A. M. Ali, Antitumor promoting and antioxidant activities of anthraquinones isolated from the cell suspension culture of Morinda elliptica, Asia Pac. J. Mol. Biol. Biotechnol, 2003, 11(1), 3-7.
- N. Ishak, L. S. Yazan, and N. H. Lajis, Nordamnacanthal induced apoptosis and mitotic-G2/M arrest with downregulation of Bcl-2 in the human breast cancer cell line (MCF-7), Med. Health Sci. J., 2010, 2, 27-39.
- L. Hassan, A. Pinon, Y. Limami, J. Seeman, C. Fidanzi-Dugas, F. Martin, B. Badran, A. Simon, and B. Liagre, Resistance to ursolic acid-induced apoptosis through involvement of melanogenesis and COX-2/PGE2 pathways in human M4Beu melanoma cancer cells, Exp. Cell Res., 2016, 345 (1), 60-69.
- L. Woźniak, S. Skąpska and K. Marszalek, Ursolic Acid-A Pentacyclic Triterpenoid with a Wide Spectrum of Pharmacological Activities, Molecules, 2015, 20 (11), 20614-20641, DOI 10.3390/molecules201119721.
- I. Baglin, A.C. Mitaine-Offer, M. Nour, K. Tan, C. Cavé, M.A. Lacaille-Dubois, A review of natural and modified betulinic, ursolic and echinocystic acid derivatives as potential antitumor and anti-HIV agents, Mini-Reviews in Medicinal Chemistry, 2003, 3, 525-539.
- I. Baglin, A.C. Mitaine-Offer, M. Nour, K. Tan, C. Cavé, M.A. Lacaille-Dubois, A.Poumaroux,, K. Tan, M. Nour, A.C. Mitaine-Offer, M.A. Lacaille-Dubois, B. Chauffert and C. Cavé, New Ursolic and Betulinic Derivatives as Cytotoxic Agents, Journal of Enzyme Inhibition and Medicinal Chemistry, 2003, 18, 111-117.
- S.-L. Deng, I. Baglin, M. Nour, O. Flekhter, C. Vita, C. Cavé, Synthesis of Ursolic Phosphonate Derivatives as Potential Anti-HIV Agents Phosphorus, Sulfur and Silicon and the Related Elements, 2007, 182, 951-967.
- N. Bakhtiari, E. Moslemee-jalalvand, J. Kazemi, Ursolic acid: a versatile triterpenoid compound in regulating the aging, Physiol. and Pharmacol, 2017, 21, 15-24.
DOI: http://dx.doi.org/10.13171/mjc65/01710131601-gassama
Refbacks
- There are currently no refbacks.
Copyright (c) 2017 Mediterranean Journal of Chemistry
