Thermal aromatic Claisen rearrangement and Strecker reaction of alkyl(allyl)-aryl ethers under green reaction conditions: Efficient and clean preparation of ortho-allyl phenols (naphthols) and alkyl(allyl)oxyarene-based γ-amino nitriles
Abstract
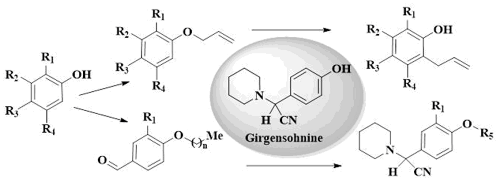
Chemical transformations of 13 diverse allyl(alkyl)-aryl ethers, easily prepared using Williamson reaction of different hydroxyarenes and allyl bromide and alkyl (n-butyl, n-octyl) bromides, were studied. Thermal aromatic Claisen rearrangement of allyl-aryl ethers to obtain ortho-allyl phenols (naphthols) employing propylene carbonate as a nontoxic and biodegradable solvent was described for the first time. The use of this green solvent allowed to enhance notably product yields and reduce significantly the reaction time comparing with the use of 1,2-dichlorobenzene, toxic solvent, which is traditionally employed in this type of Claisen rearrangement. Three-component Strecker reaction of selected alkyl(allyl)-aryl ethers with formyl function on aryl fragment, piperidine and potassium cyanide in the presence of sulfuric acid supported on silica gel (SSA, SiO2-O-SO3H) under mild reaction conditions was used in the preparation of new γ-amino nitriles, analogues of alkaloid girgensohnine [2-(4-hydroxyphenyl)-2-(piperidin-1-yl)acetonitrile], a perspective biological model in the search for new insecticidal agrochemicals against Aedes aegypti. The use of SSA, an inexpensive and reusable solid catalyst, allowed to obtain new series of 2-[4-alkyl(allyl)oxyphenyl]-2-(piperidin-1-yl)acetonitriles in short time at room temperature with good yields.
Full Text:
PDFReferences
- The Chemistry of Phenols; ed. by Z. Rappoport; WileyVCH: New York, 2003, 1605 p.
- N. Balasundram, K. Sundram, S. Samman, Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses, Food Chem., 2006, 99, 191-203.
- J. H. P. Tyman, Synthetic and Natural Phenols; Elsevier: New York, 1996, 699 p.
- Y. M. Shafran, V. A. Bakulev, V. S. Mokrushin, Synthesis and properties of γ-Aminonitriles, Russ. Chem. Rev., 1989, 58, 148-162.
- N. Otto, T. Opatz, Heterocycles from γ-Aminonitriles. Chem. Eur. J., 2014, 20, 1-15.
- N. K. Yurashevsky, N. L. Stepanova, Investigation of alkaloids of Girgensohnia oppositiflora, J. Gen. Chem. USSR, 1946, 16, 141-144.
- L. Y. Vargas-Méndez, V. V. Kouznetsov, First Girgensohnine Analogs Prepared Through InCl3-catalyzed Strecker Reaction and their Bioprospection. Curr. Org. Synth., 2013, 10, 969-973.
- A. L. Carreño Otero, L. Y. Vargas-Méndez, L. J. E Duque, V. V. Kouznetsov, Design, synthesis, acetylcholinesterase inhibition and larvicidal activity of girgensohnine analogs on Aedes aegypti, vector of dengue fever, Eur. J. Med. Chem., 2014, 78, 392-400.
- A. M. M. Castro, Claisen Rearrangement over the Past Nine Decades, Chem. Rev., 2004, 104, 2939-3002.
- K. C. Majumdar, S. Alam, B. Chattopadhyay, Catalysis of the Claisen rearrangement, Tetrahedron, 2008, 64, 597-643.
- V. H. Grant and B. Liu, Iridium(III)-catalyzed tandem Claisen rearrangement-intramolecular hydroaryloxylation of aryl allyl ethers to form dihydrobenzofuran, Tetrahedron Lett., 2005, 46, 1237-1239.
- F. C. Gozzo, S. A. Fernandes, D. C. Rodrigues, M. N. Eberlin, A. J. Marsaioli, Regioselectivity in Aromatic Claisen Rearrangements, J. Org. Chem., 2003, 68, 5493-5499.
- R. Aeschbach, J. L. Liger, B. C. Scott, A. Murcia, J. Butler, B. Halliwell, O. T. Aruoma, Antioxidant action of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol, Food Chem. Toxicol., 1994, 32, 31-36.
- M. Yamaguchi, Synthetic uses of phenols, In The Chemistry of Phenols; ed. by Z. Rappoport; WileyVCH: New York, 2003, pp. 661-712.
- R. O. Duthaler, Recent developments in the stereoselective synthesis of α-aminonitriles, Tetrahedron, 1994, 50, 1539-1650.
- K. Niknam, D. Saberi, M. N. Sefat, Silica-bonded S-sulfonic acid: an efficient and recyclable solid acid catalyst for the three-component synthesis of α-amino nitriles, Tetrahedron Lett., 2010, 51, 2959-2962.
- J. March, March's Advanced Organic Chemistry, Reaction, Mechanism, and Structure, 5th ed., John Wiley & Sons: New York, 2001, pp. 477-478.
- J. J. Li, Name Reactions, 2d ed., Springler: Berlin, 2003, pp. 437.
- Y. Peng and G. Song, Combined microwave and ultrasound assisted Williamson ether synthesis in the absence of phase-transfer catalysts, Green Chem., 2002, 4, 349-351.
- S. Xiao, Y. He, G. Xu, Q. Liu, Investigation on Claisen rearrangement of allyl phenyl ethers in near-critical water, Res. Chem. Intermed., 2015, 41, 3299-3305.
- J. C. Lee, J. Y. Yuk, S. H. Cho, Facile Synthesis of Alkyl Phenyl Ethers Using Cesium Carbonate, Synth. Commun., 1995, 25, 1367-1370.
- M. Kazemia, Z. Noori, H. Kohzadi. A mild and efficient procedure for the synthesis of ethers from various alkyl halides, Iran. Chem. Commun., 2013, 1, 43-50.
- S. H. Reich, M. Melnick, M. J. Pino, M. A. M. Fuhry, A. J. Trippe, K. Appelt, J. F. Davies, II, B.-W. Wu, L. Musick, J. Med. Chem., 1996, 39, 2781-2794.
- P. G. Jessop, Searching for green solvents, Green Chem., 2011, 13, 1391-1398.
- M. Sato, N. Otabe, T. Tuji, K. Matsushima, H. Kawanami, M. Chatterjee, T. Yokoyama, Y. Ikushima, T. M. Suzuki, Highly-selective and high-speed Claisen rearrangement induced with subcritical water microreaction in the absence of catalyst, Green Chem., 2009, 11, 763-766.
- J. H. Clements, Reactive Applications of Cyclic Alkylene Carbonates, Ind. Eng. Chem. Res. 2003, 42, 663-674.
- C. Beattie, M. North, P. Villuendas, Proline-Catalysed Amination Reactions in Cyclic Carbonate Solvent, Molecules, 2011, 16, 3420-3432.
- S. Kotha, K. Mandal, A. C. Deb, S. Banerjee, S. Microwave-assisted Claisen rearrangement on a silica gel support, Tetrahedron Lett., 2004, 45, 9603-9605.
- H. A. Oskooie, M. M. Heravi, A. Sadnia, F. Jannati, F.K. Behbahani, H2SO4/Silicagel: Highly Efficient Catalyst for the Synthesis of α-Aminonitriles Using Trimethysilyl Cyanide, Monatsh. Chem., 2008, 139, 22-29.
- E. D. Clarke, Beyond physical properties-application of Abraham descriptors and LFER analysis in agrochemical research, Bioorg. Med. Chem., 2009, 17, 4153-4159.
- C. Lamberth, S. Jeanmart, T. Luksch, A. Plant, Current Challenges and Trends in the Discovery of Agrochemicals, Science, 2013, 341, 742-746.
DOI: http://dx.doi.org/10.13171/mjc65/01711201245-kouznetsov
Refbacks
- There are currently no refbacks.
Copyright (c) 2017 Mediterranean Journal of Chemistry
