Synthesis and cytotoxicity of 3-amino-glycyrrhetinic acid derivatives
Abstract
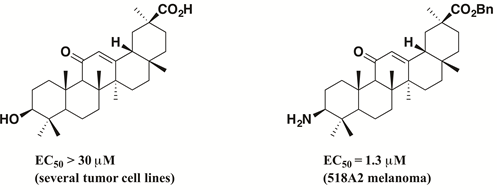
The aim of this study was to prepare 3-hydroximino- and 3-amino derivatives of glycyrrhetinic acid and derivatives to evaluate their in vitro cytotoxicity for a panel of human tumor cell lines. Thus, commercially available glycyrrhetinic acid (1) was acetylated or oxidized at position C-3 and transformed into a variety of different esters and amides followed by their conversion to 3-oximes and amines. While the parent compound was not cytotoxic at all, the 3-amino esters are highly cytotoxic. Interestingly, 3-amino amides were significantly less cytotoxic than 3-amino esters. The (3b, 18b, 20b) Benzyl 3-amino-11-oxoolean-12-en-30-oate was the most cytotoxic compound of this series showing an EC50 = 1.3 mM for 518A2 melanoma cells.
Full Text:
PDFReferences
- R. Csuk, Recent Developments in the Synthesis of Antitumor-active Glycyrrhetinic Acid Derivatives, Mini- Rev. Org. Chem., 2014, 11, 253-261.
- T.-C. Kao, C.-H. Wu, G.-C. Yen, Bioactivity and Potential Health Benefits of Licorice, J. Agric. Food Chem., 2014, 62, 542-553.
- A. Roohbakhsh, M. Iranshahy, M. Iranshahi, Glycyrrhetinic Acid and Its Derivatives: Anti-Cancer and Cancer Chemopreventive Properties, Mechanisms of Action and Structure- Cytotoxic Activity Relationship, Curr. Med. Chem., 2016, 23, 498-517.
- Z.-H. Tang, T. Li, Y.-G. Tong, X.-J. Chen, X.-P. Chen, Y.-T. Wang, J.-J. Lu, A Systematic Review of the Anticancer Properties of Compounds Isolated from Licorice (Gancao), Planta Med., 2015, 81, 1670-1687.
- R. Yang, L.-q. Wang, B.-c. Yuan, Y. Liu, The Pharmacological Activities of Licorice, Planta Med., 2015, 81, 1654-1669.
- R. Csuk, S. Schwarz, R. Kluge, D. Ströhl, Synthesis and biological activity of some antitumor active derivatives from glycyrrhetinic acid, Eur. J. Med. Chem., 2010, 45, 5718-5723.
- R. Csuk, S. Schwarz, B. Siewert, R. Kluge, D. Ströhl, Synthesis and antitumor activity of ring A modified glycyrrhetinic acid derivatives, Eur. J. Med. Chem., 2011, 46, 5356-5369.
- S. Schwarz, R. Csuk, Synthesis and antitumour activity of glycyrrhetinic acid derivatives, Bioorg. Med. Chem., 2010, 18, 7458-7474.
- S. Sommerwerk, L. Heller, C. Kerzig, A.E. Kramell, R. Csuk, Rhodamine B conjugates of triterpenoic acids are cytotoxic mitocans even at nanomolar concentrations, Eur. J. Med. Chem., 2017, 127, 1-9.
- J. Wiemann, L. Heller, R. Csuk, An access to a library of novel triterpene derivatives with a promising pharmacological potential by Ugi and Passerini multicomponent reactions, Eur. J. Med. Chem., 2018, 150, 176-194.
- B. Bednarczyk-Cwynar, A. Günther, Advances in Chemistry and Pharmacology of Triterpenoid Synthetic Dimers, Curr. Med. Chem., 2017, 24, 2205-2240.
- R. Csuk, A. Barthel-Niesen, A. Barthel, R. Schäfer, A. Al-Harrasi, 11-Keto-boswellic acid derived amides and monodesmosidic saponins induce apoptosis in breast and cervical cancers cells, Eur. J. Med. Chem., 2015, 100, 98-105.
- L. Heller, A. Knorrscheidt, F. Flemming, J. Wiemann, S. Sommerwerk, I.Z. Pavel, A. Al-Harrasi, R. Csuk, Synthesis and proapoptotic activity of oleanolic acid derived amides, Bioorg. Chem., 2016, 68, 137-151.
- B. Siewert, E. Pianowski, R. Csuk, Esters and amides of maslinic acid trigger apoptosis in human tumor cells and alter their mode of action with respect to the substitution pattern at C-28, Eur. J. Med. Chem., 2013, 70, 259-272.
- S. Sommerwerk, L. Heller, J. Kuhfs, R. Csuk, Selective killing of cancer cells with triterpenoic acid amides - The substantial role of an aromatic moiety alignment, Eur. J. Med. Chem., 2016, 122, 452-464.
- I. Beseda, L. Czollner, P.S. Shah, R. Khunt, R. Gaware, P. Kosma, C. Stanetty, M.C. del Ruiz-Ruiz, H. Amer, K. Mereiter, T. Da Cunha, A. Odermatt, D. Classen-Houben, U. Jordis, Synthesis of glycyrrhetinic acid derivatives for the treatment of metabolic diseases, Bioorgan Med Chem, 2010, 18, 433-454.
- R. Csuk, S. Schwarz, B. Siewert, R. Kluge, D. Ströhl, Conversions at C-30 of Glycyrrhetinic Acid and Their Impact on Antitumor Activity, Arch Pharm, 2012, 345, 223-230.
- L. Heller, S. Schwarz, V. Perl, A. Köwitsch, B. Siewert, R. Csuk, Incorporation of a Michael acceptor enhances the antitumor activity of triterpenoic acids, Eur. J. Med. Chem., 2015, 101, 391-399.
- B.P. Pradhan, P. Ghosh, On the study of the action of N-bromosuccinimide on triterpenoids and steroids. Part VI. Studies on the action of N-bromosuccinimide on 3-oximinolupanes in chloroform-dimethyl sulfoxide, Indian J. Chem., Sect. B, 1993, 32B, 491-493.
- T. Sundararamaiah, S.K. Ramraj, K.L. Rao, V.V. Bai, Synthesis of A-aza triterpenes. I: A-Aza triterpenes from methyl oleanonate, methyl betulonate and lupenone, J. Indian Chem. Soc., 1976, 53, 664-665.
- B. Bednarczyk-Cwynar, P. Ruszkowski, T. Bobkiewicz-Kozlowska, L. Zaprutko, Oleanolic Acid A-lactams Inhibit the Growth of HeLa, KB, MCF-7 and Hep-G2 Cancer Cell Lines at Micromolar Concentrations, Anti-Cancer Agents Med. Chem., 2016, 16, 579-592.
- B. Bednarczyk-Cwynar, N. Wachowiak, M. Szulc, E. Kaminska, A. Bogacz, J. Bartkowiak-Wieczorek, L. Zaprutko, P.L. Mikolajczak, Strong and long-lasting antinociceptive and anti-inflammatory conjugate of naturally occurring oleanolic acid and aspirin, Front. Pharmacol., 2016, 7, 201-218.
- B. Bednarczyk-Cwynar, L. Zaprutko, A. Froelich, Beckmann rearrangement of oxime obtained from oleanolic acid. Structure elucidation of the initial oxime, J. Mol. Struct., 2013, 1053, 115-121.
- B. Bednarczyk-Cwynar, L. Zaprutko, J. Marciniak, G. Lewandowski, M. Szulc, E. Kaminska, N. Wachowiak, P.L. Mikolajczak, The analgesic and anti-inflammatory effect of new oleanolic acid acyloxyimino derivative, Eur. J. Pharm. Sci., 2012, 47, 549-555.
- S. Babar, Synthesis and characterization of new imine and pthalic acid derivatives of ursolic acid, Int. J. Pharm. Pharm. Sci., 2014, 6, 560-564.
- F. Chu, W. Zhang, W. Guo, Z. Wang, Y. Yang, X. Zhang, K. Fang, M. Yan, P. Wang, H. Lei, Oleanolic Acid-amino Acids Derivatives: Design, Synthesis, and Hepatoprotective Evaluation In Vitro and In Vivo, Molecules, 2018, 23, ahead of print, doi:10.3390/molecules23020322.
- J. Wang, X. Hu, W. Wen, L. Yang, Y. Zhu, Synthesis and activity of 3-amino acid derivatives of glycyrrhetinic acid, Yingyong Huaxue, 2012, 29, 873-877.
- R. Csuk, S. Schwarz, R. Kluge, D. Ströhl, Improvement of the Cytotoxicity and Tumor Selectivity of Glycyrrhetinic Acid by Derivatization with Bifunctional Aminoacids, Arch. Pharm. 2011, 344, 505-513.
- S. Schwarz, S.D. Lucas, S. Sommerwerk, R. Csuk, Amino derivatives of glycyrrhetinic acid as potential inhibitors of cholinesterases, Bioorg. Med. Chem., 2014, 22, 3370-3378.
- C.H. Brieskorn, H. Eschelbach, Glycamines from ursolic and 18β-glycyrrhetinic acids, Arch. Pharm. 1979, 312, 752-762.
- S. Ijichi, S. Tamagaki, Molecular design of sweet tasting compounds based on 3β-amino-
β-deoxy-18β-glycyrrhetinic acid: amido functionality eliciting tremendous sweetness, Chem. Lett., 2005, 34, 356-357.
- H.-O. Kim, M.I. Goryaev, M.P. Irismetov, K.A. Alibaeva, Triterpenoids. XXVIII. Leuckart reaction with glycyrrhetic acid derivatives, Izv. Akad. Nauk Kaz. SSR, Ser. Khim., 1972, 22, 86-87.
- D.V. Krätschmar, A. Vuorinen, T. Da Cunha, G. Wolber, D. Classen-Houben, O. Doblhoff, D. Schuster, A. Odermatt, Characterization of activity and binding mode of glycyrrhetinic acid derivatives inhibiting 11β-hydroxysteroid dehydrogenase type 2, J. Steroid Biochem. Mol. Biol., 2011, 125, 129-142.
- C. Stanetty, L. Czollner, I. Koller, P. Shah, R. Gaware, T. Da Cunha, A. Odermatt, U. Jordis, P. Kosma, D. Classen-Houben, Synthesis of novel 3-amino and 29-hydroxamic acid derivatives of glycyrrhetinic acid as selective 11β-hydroxysteroid dehydrogenase 2 inhibitors, Bioorg. Med. Chem., 2010, 18, 7522-7541.
- R. Csuk, S. Schwarz, B. Siewert, R. Kluge, D. Ströhl, Synthesis and Antitumor Activity of Ring A-modified Glycyrrhetinic Acid Derivatives, Z Naturforsch B, 2011, 66, 521-532.
- X.D. Su, H. Lawrence, D. Ganeshapillai, A. Cruttenden, A. Purohit, M.J. Reed, N. Vicker, B.V.L. Potter, Novel 18 beta-glycyrrhetinic acid analogues as potent and selective inhibitors of 11 beta-hydroxysteroid dehydrogenases, Bioorgan Med Chem, 2004, 12, 4439-4457.
DOI: http://dx.doi.org/10.13171/mjc71/01804111430-cesuk
Refbacks
- There are currently no refbacks.
Copyright (c) 2018 Mediterranean Journal of Chemistry
