Characterization of scale deposits formed in sanitary hot water pipelines in the northern tourist zone of Agadir city
Abstract
The formation of scale on surfaces in contact with water is due to many reasons as the hardness of water and its temperature. Therefore, this phenomenon of scale in water pipelines is a common and inevitable problem in the regions that exploit or use groundwater with high rigidity. The circuits fed by hot water are easily reached by hard water scaling. The deposition of encrusting curst at the level of walls in touch with water is due to many technical, economic and environmental problems. It causes a reduction in water flow and a decrease in the efficiency of heating systems.
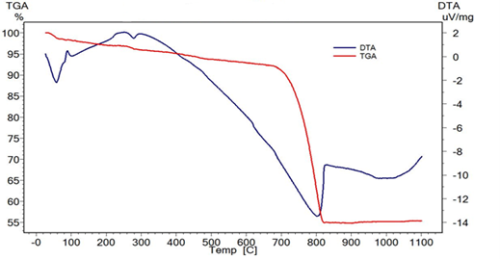
In this study, we are particularly interested in studying the phenomenon of hard water scaling caused by sanitary hot water in a tourist unit situated in the north of the seaside in the city of Agadir. First, we have evaluated the physico-chemical quality of water in use in this tourist unit. Secondly, we conducted a qualitative and quantitative analysis of the scale found in the circuits that transport sanitary hot water. Several analytical techniques were used to reach this goal namely: X-ray fluorescence (XRF) which shows that 85.50% of scale is represented by Calcium Carbonate. Whereas infrared spectrometry (IR) demonstrates the existence of the Carbonate anion CO32-. In addition, due to thermogravimetric analysis (TGA) and differential thermal analysis (DTA) we found that the endothermic event shows the decomposition of Calcium Carbonate of CaO and CO2 in the temperature range of 660 C° to 820 C°. For scanning electron microscopy (SEM), it indicates that the scale takes the form of needle-like aragonite crystals. At last, the X-ray diffraction (XRD) shows that the scale is composed essentially of Calcium Carbonate of the type aragonite.
The results of the different techniques of characterisation are in concordance in the scaling of the circuits of sanitary hot water in the tourist unit under study.
Full Text:
PDFReferences
- H.S. Ras, S. Ghizellaoui, Determination of Anti-Scale Effect of Hard Water by Test of Electrodeposition, Procedia Engineering, 2012, 33, 357-365.
- A.S. Tyusenkov, S.E. Cherepashkin, Scale Inhibitor for Boiler Water Systems. Russian Journal of Applied Chemistry, 2014, 87, 1240-1245
- N. Hafid, M. Belaatar, S. Ben-Aazza, A. Hadfi, M. Ezahri, A. Driouiche, Characterization of Scale Formed in Drinking Water and Hot Water Pipes in the Taliouine Downt, American Journal of Analytical Chemistry, 2015, 6 (8), 677-686.
- Y. Wang, Y. X. Moo, C. Chen, P. Gunawan, R. Xu, Fast precipitation of uniform CaCO3 nanospheres and their transformation to hollow hydroxyapatite nanospheres, Journal of Colloid and Interface Science, 2010, 352 (2), pp. 393-400.
- J.M. AlÃa, Y. Diaz de Mera, H.G.M. Edwards, M.P. Gonzsilez, S. Lpez Andrés, FT-raman and infrared spectroscopic study of aragonite-strontianite (CaxSr1-x,CO3) solid solution, Spectrochim. Acta Part A, 1997, 53, 2347.
- F.A. Andersen, L. Brecevie, Infrared spectra of Amorphous and Crystalline calcium Carbonate.Acta Chem.Scand, 1991,45, 1018-1024.
- G. Hongxia, Q. Zhenping, P. Qian, Y. Peng, C. Suping, W. Wei, Crystallization of aragonite CaCO3 with complex structures. Advanced Powder Technology, 2011, 22, 777-783
- A.L. Litvin, S. Valiyaveettil, D.L. Kaplan, S. Mann, Template-directed synthesis of aragonite under supramolecular hydrogen-bonded Langmuir monolayers, Adv.Mater., 1997, 9, 124.
- S. K. Abdullahi, I. Maznah, A. Tengku, I. Tengku and A.Z. Zuki. Synthesis and Characterization of Calcium Carbonate Aragonite Nanocrystals from Cockle Shell Powder (Anadara granosa), Journal of Nanomaterials, 2013, Article ID 398357, 9 pages.
- S. Huang, K. Naka, Y. Chujo, A carbonate controlled-addition method for amorphous calcium carbonate spheres stabilized by poly(acrylic acid)s, Langmuir, 2007, 23, 12086-12095.
- M. Chkir, Synthèse de gels phosphocalciques issus de déchets industriels carbonatés Caractérisation physico-chimique, thermique et rhéologique, University of Toulouse, 2011, 82-83.
- Y. Junjun, K. Xin, Q. Changlei, F. Bo, V. Ananthanarayanan, S. Dmitry, Modeling of CaCO3 decomposition under CO2/H2O atmosphere in calcium looping processes, Fuel Processing Technology, 2014, 125, 125-138.
- T. Shimizu, T. Hirama, H. Hosoda, K. Kitano, M. Inagaki, K. Tejima, A twin fluid-bed reactor for removal of CO2 from combustion processes. Chemical Engineering Research and Design, 1999, 77, 62-68.
- R. Menzri, S. Ghizellaoui, Chronoamperometry Study of the Inhibition of Groundwater Scaling Deposits in Fourchi, Energy Procedia, 2012, 18, 1523-1532.
- J.P. Nicolet, J.P. Vernet, Analyse thermique différentielle : Application au problème des carbonates. Bulletin de la Société Vaudoise des Sciences Naturelles, 1965, 69 (328), Lausanne.
- G.T. Faust, Thermal Analysis Studies on Carbonates, I-Aragonite and Calcite. The American Mineralogist, 1950, 35, 207-224.
- J.L. Bischoff, W. Hole, Temperature Controls of Aragonite-Calcite Transformation in Aqueous Solution, The American Mineralogist, 1969, 54, 149-155
- S.Knez, C. Pohar, The magnetic field influence on the polymorph composition of CaCO3 precipitated from carbonized aqueous solutions, Journal of Colloid and Interface Science, 2005, 281, 377-388.
- L.F. Wang, I. Sondi, E. Matijevic, Preparation of uniform needle-like aragonite particles by homogeneous precipitation, Journal of Colloid Interface Science, 1999, 218, 545-553
- M.M.M.G.P.G. Mantilaka, D.G.G.P. Karunaratne, R.M.G. Rajapakse, H.M.T.G.A. Pitawala, Precipitated Calcium Carbonate/Poly(Methyl Methacrylate) Nanocomposite Using Dolomite: Synthesis, characterization and properties. Powder Technology, 2013, 235, 628-632.
- T.Borch, A.K. Camper, J.A. Biederman, P.W. Butterfield, R. Gerlach, J.E. Amonette, Evaluation of Characterization Techniques for Iron Pipe Corrosion Products and Iron Oxide Thin Films, Journal of Environmental Engineering, 2008, 134, 835-844.
- N. A. Filho et al., Polymorphism of CaCO3 and microstructure of the shell of a Brazilian invasive mollusc (Limnoperna fortunei), Mat. Res., 2014, 17 (1), pp.15-22.
- A. M. Belchar, et al., Control of Crystal Phase Switching and Orientation by Soluble Mollusc-Shell Proteins, Nature, 1996, 381, pp. 56-58.
- G. T. Zhou, Q. Z. Yao, Jie ni, G. Jin, Formation of aragonite mesocrystals and implication for biomineralization American Mineralogist, 2009, 94, pp 293-302,
- J. Titschack, F. Goetz-Neunhoeffer, J. Neubauer, Magnesium quantification in calcites [(Ca,Mg)CO3] by Rietveld-based XRD analysis: Revisiting well-established method, American Mineralogist, 2011, 96, pp 1028-1038.
- J.L. Wray, F. Danniels, Precipitation of Calcite and Aragonite, Journal of the American Chemical Society, 1957, 79, 2031-2034
- Z. Hu, Y. Deng, Synthesis of needle-like aragonite from calcium chloride and sparingly soluble magnesium carbonate, Powder Technol., 2004, 140, 10-16.
DOI: http://dx.doi.org/10.13171/mjc72/01806201138-hadfi
Refbacks
Copyright (c) 2018 Mediterranean Journal of Chemistry
