Reductive amination of fusidane triterpenoid ketones
Abstract
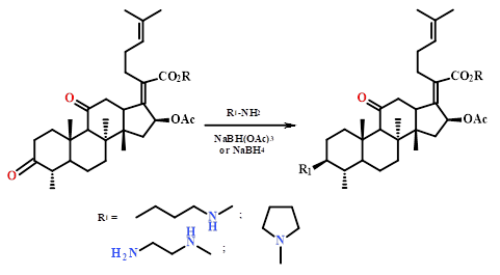
New nitrogen-containing analogues of fusidane triterpenoids were synthesized via the reductive amination of 3,11-dioxo derivatives of fusidic acid and its methyl ester by primary and secondary amines (BunNH2, Pyrrolidine, NH2CH2CH2NH2) in the presence of sodium borohydrides. The reaction proceeds with high chemo- and stereoselectivity and gives 3β-amino substituted products with yields of 75-88%.
Full Text:
PDFReferences
- S. A. Lawrence, Amines: synthesis, properties and applications, Cambridge University Press: Cambridge, 2004, pp. 371.
- R. N. Salvatore, C. H. Yoon, K. W. Jung, Synthesis of secondary amines, Tetrahedron, 2001, 57, 7785-7811.
- E. Baxter, A. Reitz, Organic Reactions; ed. by L. E. Overman; Wiley: New York, 2002, Vol. 59, pp. 1-752.
- S. Gomez, J. A. Peters, T. Maschmeyer, The reductive amination of aldehydes and ketones and the hydrogenation of nitriles: mechanistic aspects and selectivity control, Adv. Synth. Catal., 2002, 344, 1037-1057.
- J. Bodis, L. Lefferts, T. E. Muller, R. Pestman, J. A. Lercher, Activity and selectivity control in reductive amination of butyraldehyde over noble metal catalysts, Catal. Lett., 2005, 104, 23-28.
- K. A. Schellenberg, The synthesis of secondary and tertiary amines by borohydride reduction, J. Org. Chem., 1963, 28, 3259-3261.
- J. Tadanier, R. Hallas, J. R. Martin, R. S. Stanaszek, Observations relevant to the mechanism of the reductive aminations of ketones with sodium cyanoborohydride and ammonium acetate, Tetrahedron, 1981, 37, 1309-1316.
- A. F. Abdel-Magid, K. G. Carson, B. D. Harris, C. A. Maryanoff, R. D. Shah, Reductive amination of aldehydes and ketones with sodium triacetoxyborohydride. Studies on direct and indirect reductive amination procedure, J. Org. Chem., 1996, 61, 3849-3862.
- V. A. Tarasevich, N. G. Kozlov, Reductive amination of oxygen-containing organic compounds, Russ. Chem. Rev., 1999, 68, 55-72.
- M. Pereyre, J.-P. Quintard, A. Rahm, Tin in organic synthesis; Butterworths: London, 1987, p 6.
- R. E. Lenga, Ed. The Sigma-Aldrich Library of Chemical Safety Data, 1st ed.; Sigma-Aldrich Corp.: Milwaukee, 1985, p 1609.
- M. Zhao, T. Gödecke, J. Gunn, J.A. Duan, C.T. Che, Protostane and fusidane triterpenes: a mini-review, Molecules, 2013, 18, 4054-4080
- D. J. Newman, G. M. Cragg, Natural Products as sources of new drugs over the 30 years from 1981 to 2010, J. Nat. Prod., 2012, 75, 311-335.
- T. Duvold. Blanched polyamine steroid derivatives. Patent WO 03/087121 A1, 2003.
- T. Duvold. Novel fusidic acid derivatives. Patent WO 02/077007 A2, 2002.
- G. W. Gribble, C. F. Nutaitis, Sodium borohydride in carboxylic acid media. A review of the synthetic utility of acyloxyborohydrides, Org. Prep. Proced. Int., 1985, 17, 317-384.
- A. F. Abdel-Magid, C. A. Maryanoff, In reductions in organic synthesis, Chapter 12, Use of sodium triacetoxyborohydride in reductive amination of ketones and aldehydes; ACS Symposium Series; American Chemical Society: Washington, DC, 1996.
- B. Miriyala, S. Bhattacharyya, J. S. Williamson, Chemoselective reductive alkylation of ammonia with carbonyl compounds: synthesis of primary and symmetrical secondary amines, Tetrahedron, 2004, 60, 1463-1471.
- C. Salmi, Y. Letourneux, J. M. Brunel, Efficient synthesis of various secondary amines through a titanium (IV) isopropoxide-mediated reductive amination of ketones, Letters in Organic Chemistry, 2006, 3, 396-401.
- C. Salmi, Y. Letourneux, J. M. Brunel, Efficient diastereoselective titanium (IV) reductive amination of ketones, Letters in Organic Chemistry, 2006, 3, 384-389.
- C. Loncle, C. Salmi, Y. Letourneux, J. M. Brunel, Synthesis of new 7-aminosterol squalamine analogues with high antimicrobial activities through a stereoselective titanium reductive amination reaction, Tetrahedron, 2007, 63, 12968-12974.
- C. Salmi, C. Loncle, N. Vidal, Y. Letourneux, J. M. Brunel, New stereoselective titanium reductive amination synthesis of 3-amino and polyaminosterol derivatives possessing antimicrobial activities, European Journal of Medicinal Chemistry, 2008, 43, 540-547.
DOI: http://dx.doi.org/10.13171/mjc7318106921-salimova
Refbacks
- There are currently no refbacks.
Copyright (c) 2018 Mediterranean Journal of Chemistry
