Synthesis, structural characterization and ionic conductivity of mixed alkali titanium phosphate glasses
Abstract
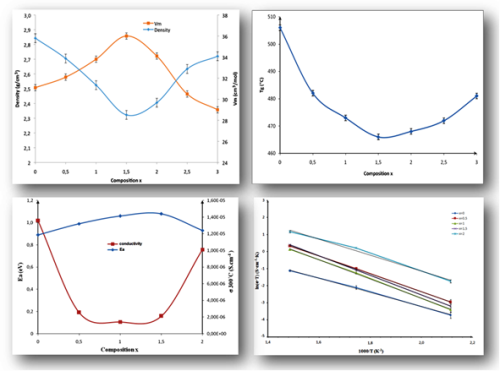
Glasses with formula Na3-xLixCaTi(PO4)3 [10(3-x) mol. % Na2O - 10x mol. % Li2O - 20 mol. % CaO - 20 mol. % TiO2 - 30 mol. % P2O5] (0 ≤ x ≤ 3) were prepared by standard melt-quenching technique, and their structural and physical properties were characterized by thermal analysis, density measurements, Raman, and impedance spectroscopy. When Na+ is gradually replaced by Li+ , molar volume, glass transition temperature (Tg) and ionic conductivity values decrease, pass through a minimum around the composition x = 1.5, then increase, while density values increase, pass through a maximum, then decrease. The non-linear variation of these physical properties is a result of the classical mixed alkali effect. Powder X-ray diffraction shows that crystallization of the glasses leads to the formation of a Nasicon phase for the compositions x = 0 and x = 0.5, and to a mixture of phases for the other compositions. Raman spectroscopy study shows that the glass structure contains P2O7 and PO4 groups, and short -Ti-O-Ti-O-Ti- chains, formed by TiO6 octahedra linked to each other through corners. These chains are linked by phosphate tetrahedra to form -O-Ti-O-P-O- linkages.
Full Text:
PDFReferences
- M. Minakshi, D. Mitchell, R. Jones, F. Alenazey, T. Watcharatharapong, S. Chakraborty, R. Ahuja, Synthesis, structural and electrochemical properties of sodium nickel phosphate for energy storage devices, Nanoscale, 2016, 8, 11291-11305.
- V. Rajendran, A. V. Gayathri Devi, M. Azooz, F. H. El-Batal, Physicochemical studies of phosphate based P2O5–Na2O–CaO–TiO2
glasses for biomedical applications, J. NonCryst. Solids, 2007, 353, 77-84.
- N. Lyczko, A. Nzihou, P. Sharrock, Calcium phosphate sorbent for environmental application, Procedia Engineer., 2014, 83, 423-431.
- A. Serghini, R. Brochu, M. Ziyad, J. C. Vedrine, Synthesis, characterization and catalytic behavior of Cu0.5M2(PO4)3 (M = Zr, Sn, Ti), J. Alloy. Compd., 1992, 188, 60-64.
- A. El Jazouli, A. El Bouari, H. Fakrane, A. Housni, I. Mansouri, R. Olazcuaga, G. Le Flem, Crystallochemistry and structural study of some Nasicon-like phosphates, J. Alloy. Compd., 1997, 262-263, 49-53.
- N. Anantharamulu, K. K. Rao, G. Rambabu, B. V. Kumar, V. Radha, M. Vithal, A wide-ranging review on Nasicon type materials, J. Mater. Sci., 2011, 46, 2821-2837.
- A. Aatiq, C. Delmas, A. El Jazouli, Structural and electrochemical study of Li0.5Mn0.5Ti1.5Cr0.5(PO4)3, J. Solid. State. Chem., 2001, 1006, 169-174.
- A. Aatiq, M. Ménétrier, A. El Jazouli, C. Delmas, Structural and lithium intercalation of Mn(0.5-x)CaxTi2(PO4)3 phases (0 ≤ x ≤ 0.5),
Solid State Ionics, 2002, 150, 391-405.
- J. B. Goodenough, H. -P. Hong, J. A. Kafalas, Fast Na+-ion transport in skeleton structures, Mater. Res. Bull., 1976, 11, 203-220.
- C. Delmas, J. -C. Viala, R. Olazcuaga, G. Le Flem, P. Hagenmuller, F. Cherkaoui, R. Brochu, Ionic conductivity in Nasicon-type
phases Na1+xZr2−xLx(PO4)3 (L = Cr, In, Yb), Solid State Ionics, 1981, 3-4, 209-214
- R. Roy, D. K. Agrawal, J. Alamo, R. A. Roy, CTP-: A new structural family of near-zero expansion ceramics, Mater. Res. Bull., 1984, 19, 471-477.
- V. I. Pet’kov, E. A. Asabina, I. A. Shchelokov, Thermal expansion of Nasicon materials, Inorg. Mater, 2013, 49, 502-506.
- A. El Jazouli, A. Nadiri, J. M. Dance, C. Delmas, G. Le Flem, Relationships between structure and magnetic properties of titanium (III) Nasicon type phosphates, J. Phys. Chem. Solids, 1988, 49, 779-783.
- J. Derouet, L. Beaury, P. Porcher, R. Olazcuaga, J. M. Dance, G. Le Flem, A. El Bouari, A. El Jazouli, A new Nasicon-type phosphate: Co0.5Ti2(PO4)3 II. Simulation of optical and magnetic properties, J. Solid State Chem., 1999, 143, 230-238.
- A. Mouline, M. Alami, R. Brochu, R. Olazcuaga, C. Parent, G. Le Flem, Structural and luminescent properties of a Nasicon-type phosphate CuI0.5MnII0.25Zr2(PO4)3, J. Solid State Chem., 2000, 152, 453-459.
- Z. Jian, Y. S. Hu, X. Ji, W. Chen, Nasicon‐structured materials for energy storage, Adv. Mater., 2017, 29, 1-16
- S. Susman, C. J. Delbecq, J. A. McMillan, M. F. Roche, Nasiglass: a new vitreous electrolyte, Solid State Ionics, 1983, 9-10, 667-673.
- A. El Jazouli, Vitrification of phosphates of Nasicon-type structure, Adv. Mat. Res., 1994, 1-2, 105-114.
- C. R. Mariappan, G. Govindaraj, B. Roling, Lithium and potassium ion conduction in A3TiBP3O12 (A=Li, K; B = Zn, Cd) Nasicon-type glasses, Solid State Ionics, 2005, 176, 723-729.
- S. Krimi, A. El Jazouli, A. Lachgar, L. Rabardel, D. de Waal, J. R. Ramos-Barrado, Glass-crystal transformation of Na5−2xCaxTi(PO4)3 phosphates, Ann. Chim.-Sci. Mat., 2000, 25, 75-78.
- S. Krimi, A. El Jazouli, A. Lachgar and J. R. Ramos-Barrado, Glass-crystal transformation of Na3MgTi(PO4)3, Phosphorus Research Bulletin, 2003, 15, 142-145.
- S. Krimi, A. El Jazouli, A. Lachgar, Crystal structure of the new titanium phosphate Na3CaTi(PO4)3, Acta Cryst., 2007, 63, 291-292.
- F. E. Dardar, A. El Jazouli, A. Lachgar, M. Gross, C. Day, Vitreous and crystalline phosphates: elaboration and electrical properties, Acta Cryst., 2014, A 70, C1766.
- J. O. Isard, The mixed alkali effect in glass, J. Non-Cryst. Solids, 1969, 1, 235-261.
- D. E. Day, Mixed alkali glasses - Their properties and uses, J. Non-Cryst. Solids, 1976, 21, 343-372.
- H. Jain, H. L. Downing, N. L. Peterson, The mixed alkali effect in lithium - sodium borate glasses, J. Non-Cryst. Solids, 1984, 64, 335-349.
- J. Swenson, S. Adams, Mixed Alkali Effect in Glasses, Phys. Rev. Lett., 2003, 90, 155507-155510.
- W. R. Heffner, A Differential Thermal Analysis Apparatus for Exploring the Glass Transition, BFY Proceedings; edited by Eblen-Zayas, Behringer, and Kozminski; the American Association of Physics Teachers, 2015, pp. 36-39.
- S. Lamrhari, Z. El Khalidi, S. Krimi, M. Haddad, M. Couzi, A. Lachgar, A. El Jazouli, Synthesis and structural characterization of phosphate-based Nasiglasses Na3Ca1-xMnxTi(PO4)3 (0 ≤ x ≤ 1), J. Mater. Environ. Sci., 2018, (Accepted).
- P. Tarte, A. Rulmont, C. Merckaert-Ansay, Vibrational spectrum of nasicon-like, rhombohedral orthophosphates MIM2IV (PO4)3, Spectrochim. Acta, 1986, 42A, 1009-1016.
- R. Pikl, D. De Waal, A. Aatiq, A. El Jazouli, Vibrational spectra and factor group analysis of Mn(0.5+x)Ti(2−2x)Cr2x(PO4)3 {0≤ x≤ 0.50}, Vib. Spectrosc., 1998, 16, 137-143.
- R. Pikl, D. De Waal, A. Aatiq, A. El Jazouli, Vibrational Spectra and Factor Group Analysis of Li2xMn0.5−xTi2(PO4)3 {x= 0, 0.25, 0.50},
Mater. Res. Bull., 1998, 33, 955-961.
- D. F. Mullica, H. O. Perkins, D. A. Grossie, Structure of Dichromate-Type Lead Pyrophosphate, Pb2P2O7, J. Solid State Chem., 1986, 62, 371-376.
- S. Kaoua, S. Krimi, S. Pechev, P. Gravereau, J.P. Chaminade, M. Couzi, A. El Jazouli, Synthesis, crystal structure, and vibrational spectroscopic and UV-visible studies of Cs2MnP2O7, J. Solid State Chem., 2013, 198, 379-385.
- C. E. Bamberger, G. M. Begun, O. B. Cavin, Synthesis and characterization of sodium-titanium phosphates, Na4(TiO)(PO4)2, Na(TiO)PO4, and NaTi2(PO4)3, J. Solid State Chem., 1988, 73, 317-324.
- M. Chakir, A. El Jazouli, J. -P. Chaminade, F. Bourée, D. De Waal, New process of preparation, X-ray characterisation, structure
and vibrational studies of a solid solution LiTiOAs1−xPxO4 (0≤x≤1), J. Solid State Chem., 2006, 179, 18-28.
- R. Chen, R. Yang, B. Durand, A. Pradel, M. Ribes, A study of the mixed alkali effect by frequency-dependent conductivity in Li2O-Na2O-P2O5 glasses, Solid State Ionics, 1992, 53-56, 1194-1199.
- A. Faivre, D. Viviani, J. Phalippou, Mixed alkali effect in Li and Na aluminophosphate glasses: influence of the cation environment, Solid State Ionics, 2005, 176, 325-332.
- Y. Gao, C. Cramer, Mixed cation effects in glasses with three types of alkali ions, Solid State Ionics, 2005, 176, 2279-2284.
- A. Bunde, M. D. Ingram, P. Maass, The dynamic structure model for ion transport in glasses, J. Non-Cryst. Solids, 1994, 172, 1222-1236.
- P. Maass, Towards a theory for the mixed alkali effect in glasses, J. Non-Cryst. Solids, 1999, 255, 35-46.
- D. E. Day, Mixed alkali glasses - Their properties and uses, J. Non-Cryst. Solids, 1976, 21, 343-372.
- J. O. Isard, The mixed alkali effect in glass, J. Non-Cryst. Solids, 1969, 1, 235-261.
DOI: http://dx.doi.org/10.13171/mjc751912040810aej
Refbacks
- There are currently no refbacks.
Copyright (c) 2018 Mediterranean Journal of Chemistry
