Model study on dilute acid pretreatment of argan pulp for bioethanol production using response surface methodology
Abstract
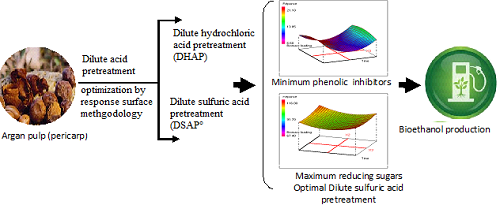
The present work describes comparative dilute acid pretreatment of the argan pulp (residue produced during the argan oil extraction) used as an economical source for bioethanol production. Response surface methodology was used to optimize the pretreatment process and to explore the effect of operational parameters (acid concentration, temperature, time and biomass loading), depending on the acid type (HCl, H2SO4) and pretreatment approach, on total and reducing sugars recovery, in addition to phenolic compounds rate as inhibitors produced during pretreatment process. Experimental results predict an optimal yield of total and reducing sugars of 171.46 mg/ml and 54.83 mg/ml, respectively, were achieved at an optimized time of 30 min with 7% of sulfuric acid at 160°C using 40 % for biomass loading.
Full Text:
PDFReferences
- M. Balat, Production of bioethanol from lignocellulosic materials via the biochemical pathway: A review. Energ. Convers. Manage, 2011 52, 858-875
- S. Prasad, A. Singh, H.C. Joshi, Ethanol as an alternative fuel from agricultural, industrial and urban residues. Resour. Conserv. Recycl., 2007, 50, 1-39.
- J.O. Metzger, A. Hüttermann, Sustainable global energy supply based on lignocellulosic biomass from afforestation of degraded areas. Naturwissenschaften, 2009, 96, 279-288. http://dx.doi.org/10.1007/s00114-008- 0479-4.
- G.A. Olah, A. Goeppert, G.K.S. Prakash, Beyond Oil and Gas: The Methanol Economy: Second Edition, Beyond Oil and Gas: The Methanol Economy: Second Edition, 2009 doi: 10.1002/9783527627806.
- M. Shaheen, M. Choi, W. Ang, Y. Zhao, J. Xing, J. Yang, J. Xing, J. Zhang, J. Chen, Application of low-intensity pulsed ultrasound to increase bioethanol production. Renew. Energy. 2013, 57, 462-468.
- W. Lim, J. Kim, H. Kim, J. Choi, I. Choi, J. Lee, Structural properties of pretreated biomass from different acid pretreatments and their effects on simultaneous saccharification and ethanol fermentation. Bioresour. Technol, 2013, 139, 214-219.
- F. Monlau, A. Barakat, E. Trably, C. Dumas, J.P. Steyer, H. Carrere, Lignocellulosic materials into biohydrogen and biomethane: impact of structural features and pretreatment. Critical. Rev. Environ. Sci. Technol, 2013, 43, 260-322.
- A. Barakat, H. de Vries, X. Rouau, Dry fractionation process as an important step in current and future lignocellulose biorefineries: a review. Bioresour. Technol, 2013, 134, 362-73.
- P. Alvira, E. Tomás-Pejó, M. Ballesteros, M.J. Negro, Pre¬treatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol, 2010, 101, 4851-4861.
- I. Ballesteros, M. Ballesteros, P. Manzanares, M.J. Negro, J.M. Oliva, F. Sáez, Dilute sulfuric acid pretreatment of cardoon for ethanol production. Biochem. Eng. J, 2008, 42, 84-91.
- Z.H. Hu, Z.Y. Wen, enhancing enzymatic digestibility of switchgrass by microwave-assisted alkali pretreatment. Biochem. Eng. J, 2008, 38, 369-378.
- C.H. Choi, B.H. Um, Y.S. Kim, K.K. Oh, Improved enzyme efficiency of rapeseed straw through the two-stage fractionation process using sodium hydroxide and sulfuric acid. Appl. Energy, 2012, 102, 640-6.
- J. Da Costa, J.E. Marques, L.R. Gonçalves, M. Valderez, Alkaline hydrogen peroxide pretreatment of cashew apple bagasse for ethanol production: a study of parameters. Bioresour. Technol, 2013,139, 249-256.
- Q. Yu, X.S. Zhuang, Z.H. Yuan, W. Qi, Q. Wang, X.S. Tan, The effect of metal salts on the decomposition of sweet sorghum bagasse in flow-through liquid hot water. Bioresour. Technol, 2011, 102, 3445-3450.
- W.H. Chen, Y.J. Tu, H.K. Sheen, Disruption of sugarcane bagasse lignocellulosic structure by means of dilute sulfuric acid pretreatment with microwave-assisted heating. Appl. Energy, 2011, 88(8), 2726-34.
- H.Z. Chen, L.Y. Liu, Unpolluted fractionation of wheat straw by steam explosion and ethanol extraction. Bioresour. Technol, 2007, 98, 666-676.
- S.Y. Jin, H.Z. Chen, Superfine grinding of steam-exploded rice straw and its enzymatic hydrolysis. Biochem. Eng. J., 2006, 30, 225-230.
- Y. Zheng, C. Lee, C. Yu, Y.S. Cheng, R. Zhang, B.M. Jenkins, Dilute acid pretreatment and fermentation of sugar beet pulp to ethanol. Appl. Energy, 2013, 105, 1-7.
- T. Vancov, S. McIntosh, Mild acid pretreatment and enzyme saccharification of Sorghum bicolor straw. Appl. Energy, 2012, 92, 421-8.
- K. Karimi, G. Emtiazi, M.J. Taherzadeh, Ethanol production from dilute-acid pretreated rice straw by simultaneous saccharification and fermentation with Mucor indicus, Rhizopus oryzae, and Saccharomyces cerevisiae. Enzyme. Microbiol. Technol, 2006, 40, 138-144.
- A.H. Brennan, W. Hoagland, D.J. Schell, High temperature acid hydrolysis of biomass using an engineering-scale plug flow reactor: Result of low solids testing. Biotechnol. Bioeng. Symp, 1986, 17, 53-70.
- A. Esteghlalian, A.G. Hashimoto, J.J. Fenske, M.H. Penner, Modeling and optimization of the dilute-sulfuric-acid pretreatment of corn stover, poplar and switchgrass. Bioresour. Technol, 1997, 59, 129-136.
- H.B. Klinke, A.B. Thomsen, B.K. Ahring, Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl. Microbiol. Biotechnol, 2004, 66, 10-26.
- S. G ámez, J.J. Gonz ález-Cabriales, J.A. RamÃrez, G. Garrote, Study of the hydrolysis of sugar cane bagasse using phosphoric acid. J. Food Eng, 2006, 74, 78-88.
- Y. Kim, E. Ximenesa, N.S. Mosiera, M.R. Ladisch, Soluble inhibitors/deactivators of cellulase enzymes from lignocellulosic biomass, Enzyme. Microb. Technol, 2011, 48 (5), 408-415.
- E.A. Ximenes, Y. Kim, N.S. Mosier, B.S. Dien, M. R. Ladisch, Inhibition of cellulases by phenols. Enzyme. Microb. Tech, 2010, 46(4), 170-176.
- E.A. Ximenes, Y. Kim, N.S. Mosier, B.S. Dien, M. R. Ladisch, Deactivation of cellulases by phenols, Enzyme. Microb. Tech, 2011, 48 (1), 54-60.
- S.F. Chen, R.A. Mowery, C.J. Scarlata, C.K. Chambliss, Compositional analysis of water-soluble materials in corn stover. J. Agr. Food. Chem, 2007, 55(15), 5912-5918.
- S. Larsson, A. Quintana-Sáinz, A. Reimann, N.O. Nilvebrant, L.J. J önsson, Influence of lignocellulose-derived aromatic compounds on oxygen-limited growth and ethanolic fermentation by Saccharomyces cerevisiae, Appl. Biochem. Biotech, 2000, 84, 617-632.
- T.A. Clark, K.L. Mackie, Fermentation inhibitors in wood hydrolysates derived from the softwood Pinus radiata, J. Chem. Technol. Biot, 1984, 34(2), 101-110.
- S.V. Sandhya, K. Kiran, M. Kuttiraja, V.E. Preeti, R. Sindhu, S. Vani, S.R. Kumar, A. Pandey, P. Binod, Evaluation of polymeric adsorbent resins for efficient detoxification of liquor generated during acid pretreatment of lignocellulosic biomass. Indian J. Exp. Biol, 2013, 51, 1012-1017.
- J. Travis, B.B.C. Lybbert, H. Narjisse, Market-based conservation and local benefits: the case of argan oil in Morocco. Ecol. Econ, 2002, 4, 125-144.
- U. Swenson, A. Anderberg, Phylogeny character evolution and classification of Sapotaceae (Ericales). Cladistics, 2005, 21, 101-130.
- 34. A. El aich, A. Bourbouze, P. Bas, P.Morand-fehr, Natural products for upgrading sustinability of land resources and landscapes : the case of the argan forest. Livestock Farming Systems: Product Quality Based on Local Resources Leading to improved sustainability, EAAP publication No.118, 2006, Benevento, Italy
- AOAC Official Methods of Analysis, 15th edn, 1990. Association of Official Analytical Chemists Washington, Dc.
- M. Dubois, K.A. Gilles, J.K. Hamilton, P.A. Rebers, F. Smith, Colorimetric method for determination of sugar and related substances. Anal. Chem, 1956, 28, 350-356.
- G.L. Miller, Use of DinitrosaIicyIic Acid Reagent for Determination of Reducing Sugar. Anal. Chem, 1959, 31, 426-428.
- V.L. Singleton, J.A. Rossi, Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic, 1965, 16, 144-158.
- T. Hatano, H.T. Kagawa, T. Okuda, Two new flavonoids and other constituent in licore root: their relative astringency and radical scavenging affects. Chem. Pharm. Bull, 1988, 36, 1090-2097.
- F.Z. Zouhair, A. Benali, M.R. Kabbour, K. El Kabous, E.H el Maadoudi, M. Bouksaim, A. Essamri, Typical characterization of argan pulp of various Moroccan areas: A new biomass for the second generation bioethanol production. Journal of the Saudi Society of Agricultural Sciences, 2018, https://doi.org/10.1016/j.jssas. 2018.09.004.
- H. Keweloh, G. Weyrauch, H.J. Rehm, Phenol-induced membrane changes in free and immobilized Escherichia coli, Appl. Microbiol. Biot, 1990, 33 (1), 66-71.
- A. Tejirian, F. Xu, Inhibition of enzymatic cellulolysis by phenolic compounds. Enzyme. Microb. Tech, 2011, 48 (3), 239-247.
DOI: http://dx.doi.org/10.13171/mjc841906025fzz
Refbacks
- There are currently no refbacks.
Copyright (c) 2019 Mediterranean Journal of Chemistry
