Synthesis, Characterization and Biological Evaluation of Benzimidazole - Dihydroartemisinin Hybrids as Potential Dual Acting Antimalarial Agents
Abstract
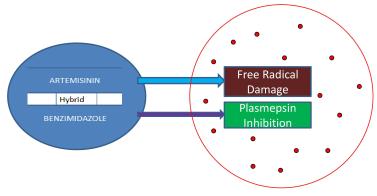
Malaria is a parasitic disease caused by various species of the Plasmodium parasite. In 2016, there were about 216 million cases resulting in 445,000 deaths, with sub-saharan Africa bearing the heaviest burden of the disease. The currently recommended treatment for malaria are combination therapies containing Artemisinin (ACT’s). However, the effectiveness of the Artemisinins is being compromised by the emergence of resistance to the drug and this amplifies the need for new antimalarial drugs. The Benzimidazole scaffold is one of the privileged structures in medicinal chemistry and is associated with a number of biological activities including antimalarial activity which may be through inhibition of the Plasmodial plasmepsin II enzyme. The present study utilizes the concept of molecular hybridization to synthesize hybrid compounds that contain two pharmacophores, acting through two distinct mechanisms. The aim is to improve efficacy and possibly prevent or slow down the emergence of parasite resistance. To confirm their structures, the conjugates were purified by chromatography and characterized using Nuclear Magnetic Resonance (NMR), Mass spectrometry and Infra-red spectroscopy. Antimalarial activities of the hybrids were evaluated in-vitro against the 3D7 strain of Plasmodium falciparum using the parasite Lactate dehydrogenase assay. The hybrids were successfully synthesized with yields ranging from 63.48 percent to 67.60 percent and were all active against the parasite. The Mebendazole conjugate of dihydroartemisinin showed the highest activity with IC50 of 6.861 nM and 6.967 nM for the 5-Benzimidazolecarboxylic acid conjugate of dihydroartemisinin. All the compounds showed statistically significant (p < 0.05) increase in activity as compared to Dihydroartemisinin and Chloroquine alone. These hybrid compounds with improved physicochemical and pharmacological properties may serve as templates for the development of a new class of anitmalarial drugs, which possess advantages over existing drugs in terms of effectiveness and also the ability to overcome the problem of resistance during malaria chemotherapy.
Full Text:
PDFReferences
- World Health Organization, World Malaria Report, WHO: Geneva, 2017, pp 2.
- R. Barnajee and D.E. Goldberg, In The Plasmodium Food Vacuole: Antimalarial Chemotherapy Mechanisms of Action, Resistance, and New Directions in Drug Discovery; ed. by P.J. Rosenthal; Humana Press: New Jersey, 2001, pp. 43-64.
- P.J. Rosenthal, Hydrolysis of erythrocyte proteins by proteases of malaria parasites, Current Opinion in Hematology, 2002, 9(2), 140-145.
- M.J. Blackman, Proteases Involved in Erythrocyte Invasion by the Malaria Parasite Function and Potential as Chemotherapeutic Targets, Current Drug Targets, 2000,1(1), 59-83.
- R. Banerjee, J. Liu, W. Beatty, L. Pelosof, M. Klemba, D.E. Goldberg, Four plasmepsins are active in the Plasmodium falciparum food vacuole, including a protease with an active-site histidine, Proceedings of the National Academy of Science U S A., 2002, 99(2), 990-995.
- K.A. Kolakovich, I.Y. Gluzman, K.L. Duffin, D.E. Goldberg, Generation of hemoglobin peptides in the acidic digestive vacuole of Plasmodium falciparum implicates peptide transport in amino acid production, Molecular and Biochemical Parasitology, 1997, 87(2),
-135.
- T.S. Haque, A.G. Skillman, C.E. Lee, Potent, Low-Molecular-Weight Non-Peptide Inhibitors of Malarial Aspartyl Protease Plasmepsin II, Journal of Medicinal Chemistry, 1999, 42(8), 1428-1440.
- D. Nöteberg, E. Hamelink, J. Hultén, Design and Synthesis of Plasmepsin I and Plasmepsin II Inhibitors with Activity in Plasmodium falciparum-Infected Cultured Human Erythrocytes, Journal of Medicinal Chemistry, 2003, 46(5), 734-746.
- D. Muthas, D. Nöteberg, Y.A. Sabnis, Synthesis, biological evaluation, and modeling studies of inhibitors aimed at the malarial proteases plasmepsins I and II, Bioorganic and Medicinal Chemistry, 2005, 13(18), 5371-5390.
- K. Jaudzems, K. Tars, G. Maurops, Plasmepsin inhibitory activity and structure-guided optimization of a potent hydroxyethylamine-based antimalarial hit, ACS Medicinal Chemistry Letters, 2014, 5(4), 373-377.
- K. Esmark, I. Feierberg, S. Bjelic, Potent inhibitors of the Plasmodium falciparum enzymes plasmepsin I and II devoid of cathepsin D inhibitory activity, Journal of Medicinal Chemistry, 2004, 47(1),110-122.
- G.H. Coombs, D.E. Goldberg, M. Klemba,
C. Berry, J. Kay, J.C. Mottram, Aspartic proteases of Plasmodium falciparum and other parasitic protozoa as drug targets, Trends in Parasitology, 2001, 17(11), 532-537.
- S. Jiang, S.T. Prigge, L. Wei L, New class of small nonpeptidyl compounds blocks Plasmodium falciparum development in vitro by inhibiting plasmepsins, Antimicrobial Agents and Chemotherapy, 2001, 45(9), 2577-2584.
- S. Romeo, M. Dell’Agli, S. Parapini, Plasmepsin II inhibition and antiplasmodial activity of Primaquine-Statine “double-drugs.†Bioorganic and Medicinal Chemistry Letters, 2004, 14(11), 2931-2934.
- P.O. Johansson, Y. Chen, A.K. Belfrage, Design and Synthesis of Potent Inhibitors of the Malaria Aspartyl Proteases Plasmepsin I and II, Use of Solid-Phase Synthesis to Explore Novel Statine Motifs Journal of Medicinal Chemistry, 2004, 47(13), 3353-3366.
- J. Camacho, A. Barazarte, N. Gamboa, Synthesis and biological evaluation of benzimidazole-5-carbohydrazide derivatives as antimalarial , cytotoxic and antitubercular agents, Bioorganic Medicinal Chemistry, 2011, 19, 2023-2029.
- K.F. Ansari, C. Lal, Synthesis, physicochemical properties and antimicrobial activity of some new benzimidazole derivatives, European Journal of Medicinal Chemistry, 2009, 44(10), 4028-4033.
- J. Cheng, J. Xie, X. Luo, Synthesis and antiviral activity against Coxsackie virus B3 of some novel benzimidazole derivatives, Bioorganic and Medicinal Chemistry Letters, 2005, 15(2),
-269.
- H. Torres-Gómez, E. Hernández-Núñez, I. León-Rivera, Design, synthesis and in vitro antiprotozoal activity of benzimidazole-pentamidine hybrids, Bioorganic and Medicinal Chemistry Letters, 2008, 18(11), 3147-3151.
- S.M. Sondhi, N. Singh, A. Kumar, O. Lozach,
L. Meijer, Synthesis, anti-inflammatory, analgesic and kinase (CDK-1, CDK-5 and GSK-3) inhibition activity evaluation of benzimidazole/benzoxazole derivatives and some Schiff’s bases, Bioorganic and Medicinal Chemistry, 2006,14(11), 3758-3765.
- Shah DI, Sharma M, Bansal Y, Bansal G, Singh M. Angiotensin II – AT1 receptor antagonists: Design, synthesis and evaluation of substituted carboxamido benzimidazole derivatives, European Journal of Medicinal Chemistry, 2008, 43(9), 1808-1812.
- N.S. El-Gohary, M.I. Shaaban, Synthesis, antimicrobial, antiquorum-sensing and antitumor activities of new benzimidazole analogs, European Journal of Medicinal Chemistry, 2017, 137, 439-449.
- M.M. Ramla, M.A. Omar, A.M.M. El-Khamry, H.I. El-Diwani, Synthesis and antitumor activity of 1-substituted-2-methyl-5-nitrobenzimidazoles, Bioorganic and Medicinal Chemistry, 2006, 14(21), 7324-7332.
- Y. Bansal, O. Silakari, The therapeutic journey of benzimidazoles: A review, Bioorganic and Medicinal Chemistry, 2012, 20(21), 6208-6236.
- N. White, M. van Vugt, F. Ezzet, Clinical pharmacokinetics and pharmacodynamics and pharmacodynamics of artemether-lumefantrine, Clinical Pharmacokinetics, 1999, 37(2),105-125.
- A.M. Dondorp, F. Nosten, P. Yi, Artemisinin Resistance in Plasmodium falciparum Malaria, New England Journal of Medicine, 2009, 361(5), 455-467.
- H. Noedl, Y. Se, K. Schaecher, B.L. Smith,
D. Socheat, M.M. Fukuda, Evidence of Artemisinin-Resistant Malaria in Western Cambodia, New England Journal of Medicine, 2008, 359(24), 2619-2620.
- A.P. Phyo, S. Nkhoma, K. Stepniewska, Emergence of artemisinin-resistant malaria on the western border of Thailand : a longitudinal study, Lancet, 2012, 379(9830), 1960-1966.
- C. Wongsrichanalai, S.R. Meshnick, Declining Artesunate-Mefloquine Efficacy against Falciparum Malaria on the Cambodia–Thailand Border, Emerging Infectious Diseases, 2008, 14(5), 716-719.
- S. Borrmann, P. Sasi, L. Mwai, Declining Responsiveness of Plasmodium falciparum Infections to Artemisinin-Based Combination Treatments on the Kenyan Coast, PLoS One, 2011, 6(11), 26005.
- F. Ariey, B. Witkowski, C. Amaratunga, A molecular marker of artemisinin-resistant Plasmodium falciparum malaria, Nature, 2014, 505(7481), 50-55.
- T.N.C. Wells, P.L. Alonso, W.E. Gutteridge, New medicines to improve control and contribute to the eradication of malaria, Nature Reviews Drug Discovery, 2009, 8(11), 879-891.
- J. Walsh, A. Bell, Hybrid Drugs for Malaria, Current Pharmaceutical Design, 2009, 15(25), 2970-2985.
- J. Walsh, D. Coughlan, N. Heneghan, C. Gaynor, A. Bell, A novel artemisinin-quinine hybrid with potent antimalarial activity, Bioorganic and Medicinal Chemistry Letters, 2007,17(13), 3599-3602.
- M.C. Lombard, D.D. N'Da, J.C. Breytenbach, P.J. Smith, C.A. Lategan, Artemisinin – quinoline hybrid-dimers : Synthesis and in vitro antiplasmodial activity, Bioorganic and Medicinal Chemistry Letters, 2010, 20(23), 6975-6977.
- M.C. Lombard, D.D. N'Da, J.C. Breytenbach, P.J. Smith, C.A. Lategan, Synthesis , in vitro antimalarial and cytotoxicity of artemisinin-aminoquinoline hybrids, Bioorganic and Medicinal Chemistry Letters, 2011, 21(6), 1683-1686.
- M.C. Lombard, D.D. N’Da, C. Tran, Potent in vivo anti-malarial activity and representative snapshot pharmacokinetic evaluation of artemisinin-quinoline hybrids, Malaria Journal, 2013, 12(1), 71.
- D.D. N’da, J.C. Breytenbach, Synthesis and Antiplasmodial Activity of EG-Artemisinin Ethers and Artemisinin – Quinoline Hybrids, South African Journal of Chemistry, 2011, 64, 163-172.
- D.D. N’da, M.C. Lombard, J. Clark, Antiplasmodial activity and cytotoxicity of 10β-aminoquinolinylethylethers of artemisinin, Drug Res (Stuttg), 2013, 63(2), 104-108.
- T. T. Cloete, C.D. Kock, P.J. Smith, D.D. N'Da, Synthesis , in vitro antiplasmodial activity and cytotoxicity of a series of artemisinin e triazine hybrids and hybrid-dimers, European Journal of Medicinal Chemistry, 2014, 76, 470-481.
- J. Chadwick, M. Jones, A.E. Mercer, Design , synthesis and antimalarial / anticancer evaluation of spermidine linked artemisinin conjugates designed to exploit polyamine transporters in Plasmodium falciparum and HL-60 cancer cell lines, Bioorganic & Medicinal Chemistry, 2010, 18(7), 2586-2597.
- C. Singh, S. Chaudhary, S. Puri, Orally active esters of dihydroartemisinin : Synthesis and antimalarial activity against multidrug-resistant Plasmodium yoelii in mice, Bioorganic and Medicinal Chemistry Letters, 2008, 18(4),
-1441.
- C. Wu, J. Liu, X. Pan, Design, synthesis and evaluation of the antibacterial enhancement activities of amino dihydroartemisinin derivatives, Molecules, 2013, 18(6), 6866-6882.
- S. Duffy, V.A. Avery, Routine In Vitro Culture of Plasmodium falciparum: Experimental Consequences, Trends in Parasitology, 2018, 34(7), 564-575.
- V.F. Andrade-Neto, A.M. Pohlit, A.C.S. Pinto, In vitro inhibition of Plasmodium falciparum by substances isolated from Amazonian antimalarial plants, Mem Inst Oswaldo Cruz, 2007, 102(3),
-366.
- M.T. Makler, D.J. Hinrichs, Measurement of the Lactate Dehydrogenase Activity of Plasmodium falciparum as an assessment of Parasitemia, American Journal of Tropical Medicine and Hygiene, 1993, 48(2), 205-210.
- N. Supraja, T.N. Prasad, A.D. Gandhi,
D. Anbumani, P. Kavitha, R. Babujanarthanam,.Synthesis, characterization and evaluation of antimicrobial efficacy and brine shrimp lethality assay of Alstonia scholaris stem bark extract mediated ZnONPs, Biochemistry and Biophysics Reports, 2018, 14, 69-77.
- P.A. Moura, J.B. Dame, D.A. Fidock, Role of Plasmodium falciparum digestive vacuole plasmepsins in the specificity and antimalarial mode of action of cysteine and aspartic protease inhibitors, Antimicrobial Agents and Chemotherapy, 2009, 53(12), 4968-4978.
- R.K. Haynes, H.W. Chan, M.K. Cheung, C-10 Ester and Ether Derivatives of Dihydroartemisinin − 10-α Artesunate, Preparation of Authentic 10-β Artesunate, and of Other Ester and Ether Derivatives Bearing Potential Aromatic Intercalating Groups at C-10, European Journal of Organic Chemistry, 2002, 1, 113-132.
- C.A. Lipinski, F. Lombardo, B.W. Dominy, P.J. Feeney, Experimental and computational approaches to estimate solubility and permeability in drug discovery and development, Advanced Drug Delivery Reviews, 2001, 46(1), 3-26.
- G. Caron, F. Reymond, P.A. Carrupt, H.H. Girault, B. Testa, Combined molecular lipophilicity descriptors and their role in understanding intramolecular effects, Pharmaceutical Science Technology Today, 1999, 2(8), 327-335.
- K. Hofmann, In The Chemistry of Heterocyclic Compounds: Imidazole and Its Derivatives; Chemistry of Heterocyclic Compounds- A Series Of Monographs; Wiley: New York, 2009, pp. 253.
- T. T. Cloete, J.W. Breytenbach, C.D. Kock, P.J. Smith, J.C. Breytenbach, D.D. N'Da, Synthesis , antimalarial activity and cytotoxicity of 10-aminoethylether derivatives of artemisinin, Bioorganic & Medicinal Chemistry, 2012, 20(15), 4701-4709.
- Y. Li, Y. Zhu, H. Jiang, Synthesis and antimalarial activity of artemisinin derivatives containing an amino group, Journal of Medicinal Chemistry, 2000, 43(8), 1635-1640.
- R. Pfau, In Benzimidazoles: Privileged Scaffolds in Medicinal Chemistry; ed. by S. Brase; The Royal Society of Chemistry Press: Cambridge, 2016, pp. 98-114.
- G. Roman, I.E. Crandall, W.A. Szarek, Synthesis and anti-Plasmodium activity of benzimidazole analogues structurally related to astemizole, ChemMedChem, 2013, 8(11), 1795-1804.
- Z.S. Saify, M.K. Azim, W. Ahmad, New benzimidazole derivatives as antiplasmodial agents and plasmepsin inhibitors: Synthesis and analysis of structure-activity relationships, Bioorganic & Medicinal Chemistry Letters, 2012, 22(2), 1282-1286.
- A.M. Silva, A.Y. Lee, S.V. Gulnik, Structure and inhibition of plasmepsin II, a hemoglobin-degrading enzyme from Plasmodium falciparum, Proceedings of the National Academy of Science, 1996, 93(19), 10034-10039.
- R. Capela, R. Oliveira, L.M. Gonçalves, Artemisinin-dipeptidyl vinyl sulfone hybrid molecules: Design, synthesis and preliminary SAR for antiplasmodial activity and falcipain-2 inhibition, Bioorganic & Medicinal Chemistry Letters, 2009, 19(12), 3229-3232.
- R. Morphy, Z. Rankovic, Designed Multiple Ligands. An Emerging Drug Discovery Paradigm. Journal of Medicinal Chemistry, 2005, 48(21), 6523-6543.
DOI: http://dx.doi.org/10.13171/mjc91190822625ua
Refbacks
- There are currently no refbacks.
Copyright (c) 2019 Mediterranean Journal of Chemistry
