Reactivity Indices related to DFT Theory, the Electron Localization Function (ELF) and Non-Covalent Interactions (NCI) Calculations in the Formation of the non-Halogenated Pyruvic Esters in Solution
Abstract
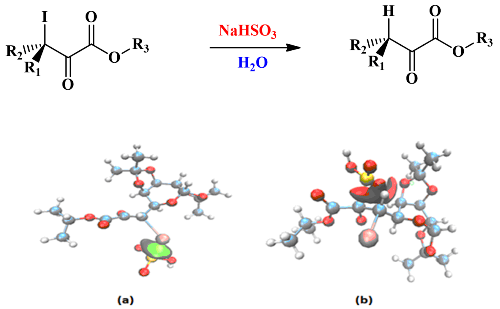
The non-halogenated pyruvic esters are essential compounds, considering that they exhibit particular properties, due to the proximity of two functional groups: carbonyl and ester. These molecules can be obtained from the approach of the Lewis acid MgI2 on the iodinated pyruvic ester by using sodium hydrogen sulfite in aqueous solution, which selectively reduces the carbon-iodine bond of the iodinated pyruvic ester. The sites of attack of hydrogen sulfite of this reaction remained uncertain and were the subject of a debate between the experimenters. Our aim in this work is to determine the more favorable sites of attack by using the local indices (Parr functions). To approve the structure of the reagents, we have conducted a topological analysis of electron localization function (ELF). To reveal the type of interaction in the "ester pyruvic-hydrogen sulfite" complex, we have performed a non-covalent interactions (NCI) calculation. The analysis of local indices and NCI analysis of electron density indicate that the approach of the hydrogen sulfite ion will take place on the iodine atom elucidating the preferable site of the attack.
Full Text:
PDFReferences
- M. A. Molenda, S. Baś, O. Elâ€Sepelgy,
M. Stefaniak, J. Mlynarski: 'Chemistry of Pyruvate Enolates: anti-Selective Direct Aldol Reactions of Pyruvate Ester with Sugar Aldehydes Promoted by a Dinuclear Zinc Catalyst', Adv. Synth. Catal., 2015, 357,
-2104.
- Y. Pocker, J.E. Meany, C. Zadorojny, Reversible hydration of pyruvate esters. Thermodynamic and kinetic studies. J. Phys. Chem., 1971, 75, 792-799.
- P. Coutrot, C. Grison, M. Tabyaoui,
S. Czernecki, et J.-M. Valery, « Novel application of alkyl dihalogenoacetates; chain extension with an α-ketoester unit of carbohydrates », J. Chem. Soc. Chem. Commun, 1988, 23, 1515-1516.
- A. Lopalco, G. Dalwadi, S. Niu, R. L. Schowen, J. Douglas, V. J. Stella, Mechanism of Decarboxylation of Pyruvic Acid in the Presence of Hydrogen Peroxide, J. Pharm. Sci., 2016, 105, 705-713.
- A.M. Ajami, C.A. Sims, M.P. Fink, Pyruvate ester composition and method of use for resuscitation after events of ischemia and reperfusion, 2005. US6846842B2.
- S.M. Reddy, S.M., University Botany- III, (Plant Taxonomy, Plant Embryology, Plant Physiology. New Age International. 2007.
- S. D. Varma, P. S. Devamanoharan, A. H. Ali: 'Prevention of Intracellular Oxidative Stress to Lens by Pyruvate and Its Ester', Free Radic. Res., 1998, 28, 131-135.
- P. Coutrot, J. C. Combert, J. Villieras, Action des organomagnesiens sur les esters glycidiques α-chlorés : préparations d'epoxycetones α- chlorés. Tetrahedron Lett., 1971, 12, 1553-1556.
- S. Jorio, H. Abou El Makarim, M. Tabyaoui: Formation of α-Iodine Pyruvics Esters from α-Chloroglycidic Esters Isomers in Diethyl Ether Solution: a DFT Study. 2018, 9, Issue 9,
-2722.
- I. J. Borowitz, S. Firstenberg, G. B. Borowitz, D. Schuessler, Organophosphorus chemistry. XVII. Kinetics and mechanism of the Perkow reaction, J. Am. Chem. Soc., 1972, 94, 1623-1628.
- M. Sekine, K. Okimoto, K. Yamada, T. Hata, Silyl phosphites.15. Reactions of silyl phosphites with alpha. halo carbonyl compounds. Elucidation of the mechanism of the Perkow reaction and related reactions with confirmed experiments, J. Org. Chem., 1981, 46, 10, 2097-2107.
- W. F. Barthel, B. H. Alexander, P. A. Giang,
S. A. Hall, Insecticidal Phosphates Obtained by a New Rearrangement Reaction, J. Am. Chem. Soc., 1955, 77, 2424-2427.
- A. D. Becke, K. E. Edgecombe, A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys, 1990, 92, 5397.
- B. Silvi, A. Savin, Classification of chemical bonds based on topological analysis of electron localization function. Nature, 1994, 371,
-686.
- G. N. Lewis, The atom and the molecule, J. Am. Chem. Soc., 1916, 38, 762-785.
- (a) S. Fiedler, J. Broecker, S. Keller, Protein folding in membranes, Cell. Mol. Life Sci. CMLS, 2010, 67, 1779-1798.
(b) K. A. Dill, Dominant forces in protein folding, Biochemistry, 1990, 29, 7133-7155.
- M. A. Spackman, E. N. Maslen, Chemical properties from the promolecular, J. Phys. Chem., 1986, 90, 2020-2027.
- A. M. Pendas, V. Luana, L. Pueyo, E. Francisco, P. Mori-Sanchez, Hirshfeld surfaces as approximations to interatomic surfaces, J. Chem. Phys., 2002, 117, 1017-1023.
- J. A. Pople, P. M. W. Gill, B. G. Johnson, Kohn-Sham density-functional theory within a finite basis, Chem. Phys. Lett., 1992, 199, 557-560.
- P.M.W. Gill, B.G. Johnson, J.A. Pople, A standard gird for density functional calculation, Chem. Phys. Lett., 1993, 209 (5-6), 506-512.
- W. Yang, Direct calculation of electron density in density-functional theory, Phys. Rev. Lett., 1991, 66, 438-1441.
- L. R. Domingo, M. J. Aurell, P. Pérez,
R. Contreras: 'Quantitative Characterization of the Local Electrophilicity of Organic Molecules. Understanding the Regioselectivity on Diels−Alder Reactions', J. Phys. Chem., A, 2002, 106, 6871-6875.
- R. G. Parr, R. A. Donnelly, M. Levy, W. E. Palke, Electronegativity: The density functional viewpoint, J. Chem. Phys., 1978, 68,
-3807.
- R. G. Parr, R. G. Pearson, Absolute hardness: companion parameter to absolute electronegativity, J. Am. Chem. Soc., 1983, 105, 7512-7516.
- R. G. Parr: in Horizons of Quantum Chemistry, eds. K. Fukui, B. Pullman, Springer Netherlands, 1980, 5-15.
- L. R. Domingo, M. José Aurell, R. Jalal,
M. Esseffar, A DFT study of the role of Lewis acid catalysts in the mechanism of the 1,3-dipolar cycloaddition of nitrile imines towards electron-deficient acryloyl derivatives, Comput. Theor. Chem., 2012, 986, 6-13.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel,
G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson and others: 'Gaussian 09, revision A. 2'.
- Dennington R., Keith T., Milam J., Gauss View, Version 5, Semichem Inc., Shwnee Mission, KS, 2009.
- D. Becke, Density-functional thermochemistry. III. The role of exact exchange, J. Chem. Phys., 1993, 98, 5648-5652.
- R. Ditchfield, W. J. Hehre, J. A. Pople, Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussianâ€Type Basis for Molecular-Orbital Studies of Organic Molecules, J. Chem. Phys., 1971, 54, 724-728.
- W. J. Hehre, R. Ditchfield, J. A. Pople, Self-Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian—Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules, J. Chem. Phys., 1972, 56, 2257-2261.
- P. C. Hariharan, J. A. Pople: 'Accuracy of AH n equilibrium geometries by single determinant molecular orbital theory', Mol. Phys., 1974, 27, 209-214.
- M. S. Gordon M. S., The isomers of Silacyclopropane. Chem. Phys. Lett., 1980, 76, 163.
- K.A. Peterson, D. Figgen, E. Goll, H. Stoll, M.J. Dolg, Systematically convergent basis sets with relativistic pseudopotentials. II. Small-core pseudopotentials and correlation consistent basis sets for the post-d group 16-18 elements. Chem. Phys., 2003, 119, 11113.
- M. Dolg, Valence correlation energies from pseudopotential calculations. Chem. Phys. Lett., 1996, 250, Issue 1, 75.-79.
- H. Tatewaki, S. Huzinaga: 'A systematic preparation of new contracted Gaussian-type orbital sets. III. Second-row atoms from Li through ne', J. Comput. Chem., 1980, 1, 205-228.
- Y. Sakai, H. Tatewaki, S. Huzinaga: 'A systematic preparation of new contracted Gaussian-type orbital sets. V. From Na through Ca', J. Comput. Chem., 1981, 2, 100-107.
- Y. Sakai, H. Tatewaki, S. Huzinaga: 'A systematic preparation of new contracted Gaussian-type orbital sets. VIII. MINI-1 and MIDI-1 sets for Ga through Cd', J. Comput. Chem., 1982, 3, 6-13.
- M. W. Wong, Vibrational frequency prediction using density functional theory, Chem. Phys. Lett., 1996, 256, 391-399.
- J. B. Foresman, T. A. Keith, K. B. Wiberg,
J. Snoonian, M. J. Frisch, Solvent Effects. 5. Influence of Cavity Shape, Truncation of Electrostatics, and Electron Correlation on ab Initio Reaction Field Calculations, J. Phys. Chem., 1996, 100, 16098-16104.
- V. Barone, M. Cossi, J. Tomasi, A new definition of cavities for the computation of solvation free energies by the polarizable continuum model, J. Chem. Phys., 1997, 107, 3210-3221.
- K. Marakchi, O. K. Kabbaj, N. Komiha, Etude DFT du mécanisme des réactions de cycloaddition dipolaire-1,3 de la C, N-diphénylnitrone avec des dipolarophiles fluorés de type éthylénique et acétylénique, J. Fluor. Chem., 2002, 114, 81-89.
- T. Lu, F. Chen, Multiwfn: A multifunctional wavefunction analyzer, J. Comput. Chem.,2012, 33, 580-592.
- J. R. Lane, J. Contreras-GarcÃa, J.P. Piquemal, B. J. Miller, H. G. Kjaergaard, Are Bond Critical Points Really Critical for Hydrogen Bonding? J. Chem. Theory Comput., 2013, 9, 3263-3266.
- E. R. Johnson, S. Keinan, P. Mori-Sánchez,
J. Contreras-GarcÃa, A. J. Cohen, W. Yang, Revealing Noncovalent Interactions, J. Am. Chem. Soc., 2010, 132, 6498-6506.
- W. Humphrey, A. Dalke, K. Schulten. VMD: Visual molecular dynamics. Journal of Molecular Graphics, 1996, 14 (1), 33-38.
- L. R. Domingo, M. T. Pisher, A DFT study of the Huisgen 1, 3-dipolar cycloaddition between hindered thiocarbonyl ylides and tetracyanoethylene, Tetrahedron, 2004, 60, 5053.
- L. R. Domingo, M. RÃos-Gutiérrez, P. Pérez, Applications of the Conceptual Density Functional Theory Indices to Organic Chemistry Reactivity, Molecules, 2016, 21(6), 748.
- L. R. Domingo, P. Pérez J. A. Sáez, Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions, RSC Adv., 2013, 3, 1486-1494.
- F. Fuster, A. Sevin B. Silvi, Topological Analysis of the Electron Localization Function (ELF) Applied to the Electrophilic Aromatic Substitution, J. Phys. Chem., A., 2000, 104, 852-858.
- M. Salah, N. Komiha, O.K. Kabbaj,
R. Ghailane, K. Marakchi, Computational study of the 1, 3-dipolar cycloaddition between methyl 2-trifluorobutynoate and substituted azides in terms of reactivity indices and activation, Journal of molecular graphics and modelling, 2017, 73, 143-151.
- A. Benallou, Z. Lakbaibi, H. Garmes, et H. E. A. El Abdallaoui, « The role of the polarity on the mechanism and selectivity in the [3+ 2] cycloaddition reaction between CF3-ynone ylide and azide group: A quantum chemical investigation », J. Fluor. Chem., 2019, 219,
-91.
- A. Benallou, Z. Lakbaibi, H. E. A. El Abdallaoui, et H. Garmes, « Understanding the molecular mechanism in a regiospecific [3+ 2] cycloaddition reaction including CO interaction: A MEDT study », RHAZES Green Appl. Chem., 2018, 3, 12, 01-12.
DOI: http://dx.doi.org/10.13171/mjc8619072612sj
Refbacks
- There are currently no refbacks.
Copyright (c) 2019 Mediterranean Journal of Chemistry
