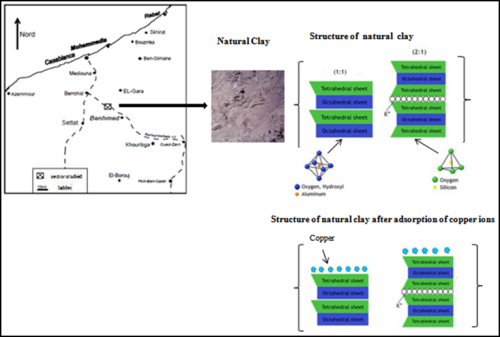
Kinetic and Thermodynamic Study of Adsorption of Copper (II) Ion on Moroccan Clay
Abstract
Full Text:
PDFReferences
- H. Ali, E. Khan, I. Ilahi, Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation, Journal of Chemistry 2019, 6730305, 14.
- M. Abbas, M. Adil, S. Ehtisham-ul-Haque,
B. Munir, M. Yameen, A. Ghaffar, G. Abbas Shar, M. Asif Tahir, Munawar Iqbal eVibrio fischeri bioluminescence inhibition assay for ecotoxicity assessment: A review, Science of the Total Environment, 2018, 626, 1295-1309.
- M. Iqbal, M. Abbas, A. Nazir , A. Zaman Qamar Bioassays based on higher plants as excellent dosimeters for ecotoxicity monitoring: A review, Iqbal et al / Chemistry International, 2019, 5, 1-80.
- M. Khajeh, A. Sarafraz-Yazdi, A. Fakhrai Moghadam, Modeling of solid-phase tea waste extraction for the removal of manganese and cobalt from water samples by using PSO-artificial neural network and response surface methodology, Arabian Journal of Chemistry, 2017, 10, 2, S1663-S1673.
- O. Chidi and R. Kelvin, Surface interaction of sweet potato peels (Ipomoea batata) with Cd(II) and Pb(II) ions in aqueous medium, Chemistry International , 2018, 4(4), 221-229.
- M. Sasmaz , E.Obek , A. Sasmaz , Bioaccumulation d'uranium et de thorium par Lemna minor et Lemna gibba dans de l'eau de ruissellement Pb-Zn-Ag, Bull. Environ. Contam. Toxicol., 2016, 97, 832-837.
- 7.M. Taseidifar, F. Makavipour, R.M. Pashley, A.M. Rahman, Removal of heavy metal ions from water using ion flotation, Environ. Technol. Innov., 2017, 8, 182-190.
- Y. Xu, J. Chen, R. Chen, P. Yu, S. Guo,
X. Wang, Adsorption and reduction of chromium(VI) from aqueous solution using polypyrrole/calcium rectorite composite adsorbent, Water Research, 2019, 160, 148-157.
- A. Kausar, G. MacKinnonb, A. Alharthic, J. Hargreaves and H.N. Bhatti and Munawar, A green approach for the Sr(II) removal from aqueous media: Kinetics, isotherms and thermodynamic studies, Journal of Molecular Liquids, 2018, 257, 164-172.
- M. Akram, H. Nawaz Bhatti, M. Iqbal, S. Noreen, S. Sadaf, Biocomposite efficiency for Cr(VI) adsorption: Kinetic, equilibrium and thermodynamics studies, 2017, 5, 1, 400-411.
- M. Debure, C. Tournassat, C. Lerouge, B. Madé, J. C Robinet, A. M. Fernández, S. Grangeon, Retention of arsenic, chromium and boron on an outcropping clay-rich rock formation (the Tégulines Clay, eastern France), Science of the Total Environment, 2018, 642, 216-229.
- A. Chham, E. H. Khouya, M. Oumam, A. Abourriche, S. Gmouh, M. Larzek, S. Mansouri, N. Elhammoudi, N. Hanafi, H. Hannache, The use of insoluble matter of Moroccan oil shale for removal of dyes from aqueous solution, Chham et al., Chemistry International, 2018, 4(1), 67-7.
- A. Kausar, M. Iqbal, A. Javed, K. Aftab, Z. Nazli, H. N. Bhatti, S. Nouren, Dyes adsorption using clay and modified clay: A review, Journal of Molecular Liquids, 2018, 256, 395-407.
- Interactions entre microorganisms et minéraux argileux: Nouvelles idées et applications plus larges, Science de l'argile appliquée,2019, 177, 91-113
- S.Mnasri-Ghnimi, N.Frini-Srasra, Removal of heavy metals from aqueous solutions by adsorption using single and mixed pillared clays, Applied Clay Science, 2019, 179, 105151
- V.Gionis, G. H. Kacande, I. D. Kastritis, G. D. Chryssikos, On the structure of palygorskite by mid- and near-infrared spectroscopy, American Mineralogist, 2006, 91, 1125-1133.
- M. Suárez, E. GarcÃa-Romero, FTIR spectroscopic study of palygorskite: influence of the composition of the octahedral sheet. Appli. Cl. Scie., 2006, 31, 154-163.
- C. Blanco, F. Gonzalez, C.Pesquera, I. Benito, S. Mendioroz, J. A. Pakhares, Differences between one palygorskite and another magnesic by infrared spectroscopy, Spectros. Lett., 1989, 22, 659-673.
- J. Madejova´ and P. Komadel Baseline studies of the clay minerals society clays: infrared methods, Clays and Clay Minerals, 2001, 49, 5, 410-432.
- S. Veli, B. Alyuz, Adsorption of copper and zinc from aqueous solutions by using natural clay J. Hazard. Mater., 2007, 149, 226-233.
- M. Zouraibi, A.Ammuri, K. Ziat, Adsorption of Cu(II) onto natural clay: Equilibrium and thermodynamic studies, Journal of Materials and Environmental Science, 2016, 7(2), 566-570
- S. Lagergren, K. Sven. Vetenskapsakad. Handl., zur Theorie der sogenannten adsorption geloter stoffe, 1898, 24, 1-39.
- Y-S. Ho, A. E. Ofomaja, Pseudo-Second-Order Model for Lead Ion Sorption from Aqueous Solutions onto Palm Kernel Fiber, J. Hazard. Mater. B, 2006, 129(1-3), 137-142.
- A. Ayach, S. Fakhi, Z. Faiz, A. Bouih, O. Ait malek, A. Benkdad, M. Benmansour, A. Laissaoui, M. Adjour, Y. Elbatal, I. Vioque, G. Manjon, Adsorption of Methylene Blue on bituminous schists from Tarfaya-Boujdour, Chemistry International, 2017, 3, 343-352.
- Turner N. H., J. Catal., 1975, 36, 262-265.
- N. Y. Mezenner, Z. Bensaadi, H. Lagha, A. Bensmaili, Larhy. J., 2012,11, 7-16.
- H. Freundlich, über die adsorption in lösungen, Zeitschrift für Physikalische Chemie Zeitsch, 1907, 57, 385-471.
- I. Langmuir, The constitution and fundamental properties of solids and liquids, J. Am. Chem. Soc, 1916, 38, 2221-2295.
- G-Z.Fang, J. Tan, X-P.Yan, An ion-imprinted functionalized silica gel sorbent prepared by a surface imprinting technique combined with a sol-gel process for selective solid-phase extraction of cadmium(II), Anal. Chem., 2005, 77, 1734-1739.
- M. M. Dubinin, L. V. Radushkevich, Equation of the Characteristic Curve of Activated Charcoal, Proceedings of the Academy of Sciences of the USSR: Physical Chemistry Section, 1947, 55, 331-337.
- N. Yeddou Mezenner, A. Bensmaili, Kinetics and thermodynamic study of phosphate adsorption on iron hydroxide-eggshell waste, The Chemical Engineering Journal, 2009, 147(2-3), 87-96.
- T.O Chime, U S Ilo, S.O Egbuna, Kinetics, Isotherms and Thermodynamic Study of Copper Adsorption On To Activated Carbon from African Bush Mango Seed Shells (Irvingia), International Journal of Engineering and Technology, 2015, 5, 11.
- A.M. Alkherraz, A. Khalifa Ali, K. M. Elsherif, Removal of Pb (II), Zn (II), Cu (II) and Cd (II) from aqueous solutions by adsorption onto olive branches activated carbon: Equilibrium and thermodynamic studies, Chemistry International, 2020, 6, 11-20.
DOI: http://dx.doi.org/10.13171/mjc92190909510he
Refbacks
- There are currently no refbacks.
Copyright (c) 2019 Mediterranean Journal of Chemistry
