NMR Investigation of the complexation of (S)-2-isopropyl- 1-(o-nitrophenyl)sulfonyl)aziridine with ï¢-cyclodextrin
Abstract
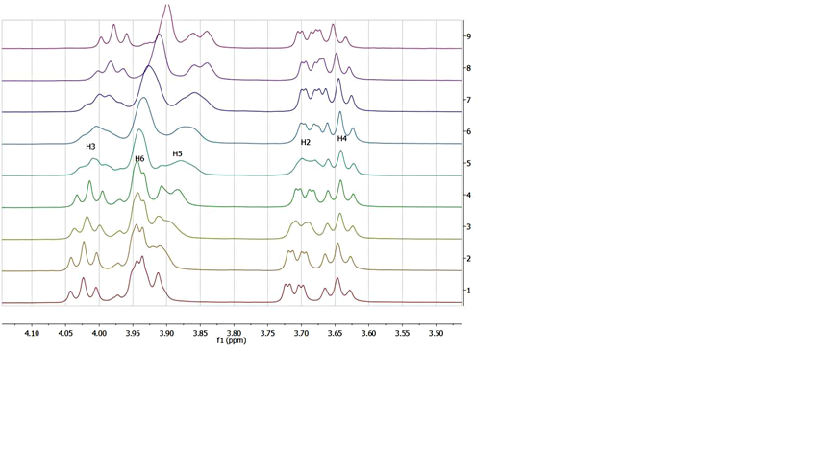
 Aziridines are known to undergo hydrolysis in the presence of cyclodextrins, whereas the latter are largely investigated as potential vectors of biologically active compounds. Despite this easy cyclodextrin-induced cleavage of aziridines in aqueous medium, it was of interest to find out a model aziridine derivative that would be sufficiently water-stable and form a stable complex with b-cyclodextrin in aqueous medium, so that it could be used as a reference in future formulations or vectorization work. Among compounds we have investigated, we found out that only (S)-2-isopropyl-1-(o-nitrophenyl)sulfonyl)aziridine complied with the above-mentioned solubility and stability requirements. NMR studies of the inclusion complex of this derivative with b-cyclodextrin provided useful parameters related to the stoichiometry of the complex and the association constant Ka. The geometry of the complex was assessed by 2D-ROESY experiments, suggesting a deep insertion of the aziridine into the cavity of b-cyclodextrin.
Full Text:
PDFReferences
- (a) J. Szejtli J. Chem. Rev., 1998, 98, 1743-1753.
(b) M. Schirra, G. Delogu, P. Cabras, A. Angioni, G. D’Hallewin, A. Veyrat, J. Agric. Food Chem., 2002, 50, 6790-6797.
- M. E. Davis, M.E. Brewster, Nature Rev. Drug Discov., 2004, 3, 1023-1035
- A. A. Desai, H. Ren, M. Mukherjee, W. D. Wulff, Org. Process Res. Dev., 2011, 15, 1108-1115.
- A. Keniche, A. Mezrai, J. Kajima Mulengi, The Open Conf. Proceed., J. 2011, 2, 28-35.
- (a) D. Kurmich, J. R. Regan, D. Disalvo PCT Int. Appl., 2009. WO 2009015067 A2 20090129. US20100048950. Application Number: 12/521005/ Publication: 02/25/2010
(b) M. D’hoodge, I. Kerkaert, M. Rottiers, N. De Kimpe Tetrahedron, 2004, 60, 3637-3641.
- (a) J. Farras, X. Giniesta, P. W. Sutton, J. Taltavull, F. Egeler, P. Romea, F. Urpi, J. Vilarrasa Tetrahedron, 2001, 57, 7665-7674.
(b) B. Moon, S. Mog So, H. Jin Choi Org. Lett., 2002, 4, 949-952.
(c) F. Crestey, M. Witt, K. Frydenvang, D. Stærk, J. W. Joroszewsky, H. Franzyk, J. Org. Chem., 2008, 73, 3566-3569.
(d) B. M. Chanda, R. Vyas, A. Bedekar J. Org. Chem., 2001, 66, 30-34.
- (a) H. Xu, H. Tian, L. Zheng, Q. Liu, L. Wang, S. Zhang Tetrahedron Lett., 2011, 52, 2873-2875.
(b) H. Rubin, J. Cockrell, J. B. Morgan, J. Org. Chem., 2013, 78, 8865-8871.
- (a) V. G. Nenajdenko, A. S. Karpov, E. S. Balenkova Tetrahedron Asymmetry, 2001, 12, 2517-2527.
(b) M. Cernerud, H. Adolfsson, C. Moberg, Tetrahedron Asymmetry, 1997, 8, 2665-2662.
(c) L. W. Bieber, M. C. F. Araujo, Molecules, 2002, 7, 902-906.
- J. Bornholdt, J. Felding, R. P. Rasmus, J. L. Kristensen, Chemistry, A European J., 2010, 16, 12474-12480.
- (a) H-L. Kwong, D. Liu, K-Y. Chan, C-S. Lee, K-H. Huang, C-M. Che Tetrahedron Lett., 2004, 45, 3965-3968.
(b) H. Kawabata, K. Omura, T. Katsuki, Tetrahedron Lett., 2006, 47, 1571-1574.
- L. Fielding, Tetrahedron, 2000, 56, 6151-6170.
- W. Medjahed, A. Tabet Zatla, J. Kajima Mulengi, F. Z. Baba Ahmed, H. Merzouk, Tetrahedron Lett., 2004, 45, 1211-1213.
- (a) S. Mashood Ali, K. Fatma, S. Dhokale, Beilstein J. Org. Chem., 2013, 9, 1917-1924
(b) R. Singh, B. Nitin, M. Jyotsana, S. N. Hiremath J. Pharm. Sciences 2010, 2, 171-183
- K. Surendra, N. Srilakshmi Krishnaveni, M. Arjun Reddy, Y. V. D. Nageswar, K. Rama Rao J. Org. Chem., 2003, 68, 9119-9121.
- (a) B. Srinivas, V. Pavan Kumar, R. Sridhar, K. Surendra, Y. V. D. Nageswar, K. Rama Rao, J. Mol. Catal. A: Chem., 2007, 261, 1-5.
(b) M. Somi Reddy, M. Narender, Y. V. D. Nageswar, K. Rama Rao, Tetrahedron Lett., 2005, 46, 6437-6439.
- G. Maatz, A. Maciollek, H. Ritter Beilstein J. Org. Chem., 2012, 8, 1929-1935
- J. H. Beijnen, S. C. van der Schoot, B. Nuijen, F. M. Flesch, A. Gore, D. Mirjovsky, L. Lenaz, Drug Devel.Indus.Pharm, 2008, 34, 1130-1139.
- K. S. Cameron, D. Fletcher, L. Fielding, Magn. Reson. Chem. 2002, 40, 251-260.
- P. Job, Ann. Chim. 1928, 9, 113-203.
- P. Job, Compt. Rend. Acad. Sci. Paris 1925, 180, 928-930.
- L. Fielding, S. McKellar, J. F. Alastair, Magn. Reson. Chem. 2011, 49, 405-412.
- (a) S. M. Ali, S. K. Upadhyay, Magn. Reson. Chem., 2008, 46, 676-679;
(b) G. Wenz, Beilstein J. Org. Chem. 2012, 8, 1890-1895.
- N. Funasaki, S. Ishikawa, S. Neya, J. Phys. Chem. B, 2003, 107, 10094-10099.
- K. Hirose, J. Incl. Phenom. Macrocycl. Chem. 2001, 39, 193-209.
- (a) H. A. Benesi, J. H. Hildebrand, J. Am Chem. Soc. 1949, 71, 2703-2707.
(b) M. W. Hanna, A. L. Ashbaugh, J. Phys. Chem. 1964, 68, 811-816.
- R. Foster, C. A. Fyfe, Trans. Faraday Soc., 1965, 61, 1626-1631.
- R. Foster, C. A. Fyfe, J. Chem. Soc., Chem. Commun. 1965, 642.
- R. L. Scott, Rec. Trav. Chim. Pays-Bas 1956, 75, 787-789.
- G. Scatchard, Ann. N. Y. Acad. Sci. 1949, 51, 660-672.
- N. J. Rose, R. S. Drago, J. Am. Chem. Soc. 1959, 81, 6138-6141.
- D. Salvatierra, C. DÃez, C. Jaime, J. Incl. Phenom., 1997, 27, 215-231.
- A. Bax, D. G. Davies, J. Magn. Reson., 1985, 65, 355-360.
- V. Laine, A. Coste-Sarguet, A. Gadelle, J. Defaye, B. Perly, F. Djedaïni-Pilard, J. Chem. Soc. Perkin Trans, 1995, 2, 1479-1481.
- F. Djedaïni-Pilard, N. Azaroual-Bellanger, M. Gosnat, D. Vernet, B. Perly, J. Chem. Soc. Perkin Trans, 1995, 2, 723-730.
- C. Pean, C. Créminon, J. Grassi, P. Pradelles, B. Perly, F. Djedaïni-Pillard, J. Incl. Phenom. Macrocycl. Chem,. 1999, 33, 307-319.
DOI: http://dx.doi.org/10.13171/mjc.2.5.2013.01.12.23
Refbacks
- There are currently no refbacks.
Copyright (c) 2015 Mediterranean Journal of Chemistry
