Evaluation of First Response ® HCV card test as a reliable rapid test for HCV detection
Abstract
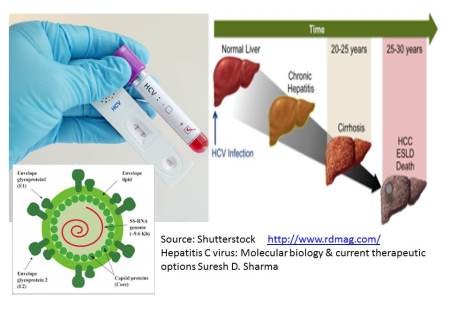
Hepatitis C Virus (HCV) infection is a global health issue causing approximatively 500000 deaths each year. Untreated, chronic HCV infection can lead to progressive hepatic fibrosis, cirrhosis, end-stage liver disease, and hepatocellular carcinoma. There is a strong need for an effective vaccine that protects against the different genotypes of HCV but the prevention still represents an enormous challenge.
HCV was first identified in 1989 using molecular methods at the Chiron Corporation, but to date, the virus has never been visualized or grown in cell culture. The general nowadays method of detecting infection with HCV is to observe the presence of antibodies to the virus by an EIA enzyme immunoassays method followed by confirmation with Western Blot. A Rapid Anti-HCV Test; based on immuno-chromatography; is a simple, visual qualitative test that detects antibodies in human serum or plasma within 15 minutes.
The objective of this work is to evaluate the performance and compare the results obtained by two HCV detection platforms with high throughput Advia Centaur XP (Siemens Healthcare Diagnostics, USA) and First Response ® HCV Card Test HCV (Premier Medical Corporation Limited).
These data could help in HCV control and understanding and hopefully, in vaccine design and development, moreover to identify a reliable test to evaluate and prevent HCV infection.
Full Text:
PDFReferences
Recep KesliAn- Overview of the Laboratory Assay Systems and Reactives Used in the Diagnosis of
Hepatitis C Virus (HCV) Infections. Trends in Immunolabelled and Related Techniques- Chapter 21
ISBN 978-953-51-0570-1 InTech- 27, April, 2012.
Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2095-2128.
El-Kamary SS, Jhaveri R, Shardell MD. All-cause, liver-related, and nonliver-related mortality among HCV-infected individuals in the general US population. Clin Infect Dis 2011; 15: 150-7.
Seeff LB. Natural history of chronic hepatitis C. Hepatology 2002; 36(5 Suppl. 1):S35-46.
Strickland GT, El-Kamary SS, Klenerman P, Nicosia A. Hepatitis C vaccine: supply and demand. Lancet Infect Dis 2008;8:379-86.
Pybus OG, Drummond AJ, Nakano T, Robertson BH and Rambaut A (2003) The epidemiology and iatrogenic transmission of hepatitis C virus in Egypt: a Bayesian coalescent approach. Mol Biol Evol 20: 381-387.
Abdelwahab S, Rewisha E, Hashem M, Sobhy M, Galal I, et al. (2012) Risk factors for hepatitis C virus infection among Egyptian healthcare workers in a national liver diseases referral centre. Trans R Soc Trop Med Hyg 106: 98–103.
Gebo KA, Jenckes MW, Chander G, Torbenson MS, Ghanem KG, Herlong HF, et al. Management of chronic hepatitis C. Evid Rep Technol Assess (Summ) 2002;60:1-7.
Kamal SM, El Kamary SS, Shardell MD, Hashem M, Ahmed IN, Muhammadi M, et al. Pegylated interferon alpha-2b plus ribavirin in patients with genotype 4 chronic hepatitis C: the role of rapid and early virologic response. Hepatology 2007; 46:1732-40.
Colao M. G., T. Capobianco, G. Mazzarelli, F. Parri Comparison of two chemiluminescent automated systems for the screening of hbsag, hcv, hiv.
Kuo, G., Q.L. Choo, H.J. Alter, and M. Houghton. An assay for circulating antibodies to a a major etiologic Virus of human non-A, non-B hepatitis. Science 1989; 244: 362.
Alter HJ, Holland PV, Morrow AG et al. Clinical and serological analysis of transfusion-associated
hepatitis. Lancet 1975;2: 838-41.
Esteban Jl, Gonzalez A. Hernandez JM et al. Evaluation of antibodies to hepatitis C virus in a study of transfusion-associated hepatitis. N Engl J Med 1990:323: 1107-12.
Siemens Healthcare Diagnostics Inc. SUMMARY OF SAFETY AND EFFECTIVENESS: ADVIA Centaur® HIV Ag/Ab Combo (CHIV) Assay Summary of Safety and Effectiveness. URL:
http://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/ PremarketApprovalsPMAs/UCM450883.pdf Date access 6.16.2016.
Immunochromatographic rapid test cassette for qualitative detection of antibody against HCV in serum- Biocare TM Diagnostics- Catalog #: ID1010C URL: http://www.ivdbiocare.com/HCV%20card.pdf Date access 6.16.2016.
Refbacks
- There are currently no refbacks.
