Review on telomerase activity in metastasis and proposal of amino-modified polystyrene-antisense human RNA nanoparticles in killing metastatic cells
Abstract
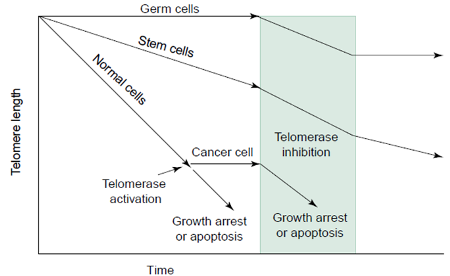
Telomeres protect chromosomes from losing base-pair sequences at their ends. Part of telomeres is lost during each cell division. When the telomere becomes too short, the chromosome can no longer replicate. Telomerase, by reverse transcription, maintains telomere length and stability in cancer, gonadal and hemopoietic stem cells. During telomerase maturation, the RNA component is transferred to the cytoplasm where it recruits proteins and the mature complex is then re-imported into the nucleus. Telomerase activity in cancer cells is 10-20 times greater than that in normal cells.
Amino silica nanoparticles are transport carrier for antisense human telomerase RNA (hTR) to cytoplasm where hTR binds to telomerase mRNA leading to its degradation. Sometimes anti-telomerase leads to growth arrest but not to cancerous cell death.
It is proposed that amino polystyrene (NH2PS) can replace amino silica nanoparticles and carry antisense hTR to the cytoplasm. After the binding of the antisense hTR to the mRNA of telomerase, NH2PS nanoparticles will be released. These nanoparticles  inhibit the mammalian target of rapamycin (mTOR) signaling, leading to G2 cell cycle arrest of all cells exhibiting activated mTOR signaling including cancer cells. Due to the absence of mTOR signaling, macrophage resist to NH2PS toxicity. Besides, NH2PS inhibits angiogenesis and proliferation of tumour cells.
Full Text:
PDFReferences
Ryungsa Kim. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007 May; 121(1): 1–14.
C. Pionneau1. Proteomic Analysis of Membrane-associated Proteins from the Breast Cancer Cell Line MCF7. Cancer Genomics & Proteomics 2, 2005, 199-208.
Facts About Telomeres and Telomerase: Shay/Wright Lab – UT Southwestern
Cassandra D. Belair. Telomerase activity: A biomarker of cell proliferation, not malignant transformation. Cell Biology. Proc. Natl. Acad. Sci. USA. Vol. 94, pp. 13677–13682, December 1997.
Laura K. White. Telomerase inhibitors. TRENDS in Biotechnology Vol.19 No.3 March 2001.
Haijia Wu. Telomerase RNA TLC1 Shuttling to the Cytoplasm Requires mRNA Export Factors and Is Important for Telomere Maintenance. Cell Reports 8, 1630–1638, September 25, 2014.
Gabriele Saretzki. Telomerase inhibition as cancer therapy. Cancer Letters 194 (2003) 209–219
Ji-yong Liu. The Retrovirus-mediated Antisense Human Telomerase RNA (hTR) Gene Limits the Growth of Hepatocellular Carcinoma Growth in Cell Culture and Animals. Dig. Dis Sci 2008, 53:1122-1130.
Run-Hua Feng. Inhibition of human telomerase in MKN-45 cell line by antisense hTR expression vector induces cell apoptosis and growth arrest. World J Gastroenterol 2002;8(3): 430-440.
RNA-induced silencing complex-Wikipedia, the free encyclopedia.
Christineantler. AntiSense RNA. The Science Creative Quaterly, August 2003.
Ana Preto. Telomere erosion triggers growth arrest but not cell death in human cancer cells retaining wild-type p53: implications for antitelomerase therapy. Oncogene 2004: 23, 4136–4145.
M Castedo. Mammalian Target of Rapamycin (mTOR): Pro- and Anti-Apoptotic. Cell Death and Differentiation (2002) 9, 99-100.
Daniela Guarnieri. Effect of serum proteins on polystyrene nanoparticle uptake and intracellular trafficking in endothelial cells. Journal of Nanoparticle Research. September 2011, Volume 13, Issue 9, pp 4295-4309.
Cornelia Loos . Functionalized polystyrene nanoparticles as a platform for studying bio–nano interactions. Beilstein J. Nanotechnol. 2014, 5, 2403–2412.
Nazila Kamaly. Paramagnetic Liposome Nanoparticles for Cellular and Tumour Imaging . Int. J. Mol. Sci. 2010, 11, 1759-1776.
Kim, Jong Ah. Low Dose of Amino-Modified Nanoparticles Induces Cell Cycle Arrest. ACS NANO, 7 (9): 7483-7494.
Jakub Stanislaw Nowak. Silica nanoparticle uptake induces survival mechanism in A549 cells by the activation of autophagy but not apoptosis. Tocicology Letters. Volume 224, Issue1, 3 January 2014, pages 84-92.
Rahul P. Bagwe. Surface modification of silica nanoparticles to reduce aggregation and non-specific binding. Langmuir. 2006 Apr 25;22(9): 4357-4362
Jing Jiang and S. Thayumanavan. Synthesis and Characterization of Amine-Functionalized Polystyrene Nanoparticles. Macromolecules, 2005, 38 (14), pp 5886–5891.
Jiaofeng Peng. An antisense oligonucleotide carrier based on amino silica nanoparticles for antisense inhibition of cancer cells. Nanomedicine: Nanotechnology, Biology and Medicine, Volume 2, Issue 2, Pages 113-120.
Cornelia Loos. Amino-functionalized nanoparticles as inhibitors of mTOR and inducers of cell cycle arrest in leukemia cells. Biomaterials. Volume 35, issue 6, February 2014, pages 1944-1953.
Amine-Reactive Probe Labeling Protocol / Thermo Fisher Scientific.
Verena Holzapfel . Preparation of Fluorescent Carboxyl and Amino Functionalized Polystyrene Particles by Miniemulsion Polymerization as Markers for Cells. Macromolecular Chemistry and Physics. Volume 206, Issue 24, pages 2440–2449, December 22, 2005.
Jani PU. Biliary excretion of polystyrene microspheres with covalently linked FITC fluorescence after oral and parenteral administration to male wistar rats. Journal of Drug Targeting. 1996; 4 (2): 87-93.
Xiao-Yang He. Efficient activation of aflatoxin B1 by cytochrome P450 2A13, an enzyme predominantly expressed in human respiratory tract. International Journal of Cancer. Volume 118, Issue 11, pages 2665–2671, 1 June 2006.
Kaplan-Meier survival analysis-MedCalc
Manish Kumar Goel. Understanding survival analysis: Kaplan-Meier estimate. Int J Ayurveda Res. 2010 Oct-Dec; 1(4): 274–278.
Refbacks
- There are currently no refbacks.
