Rotational barrier and electron-withdrawing substituent effects: Theoretical study of -conjugation in para-substituted anilines
Abstract
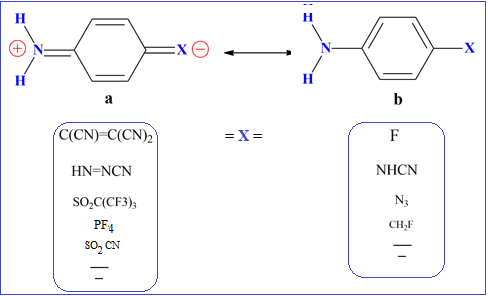
The rotational barrier RB around C–NH2 bond between the minimum and maximum states of 84 electron-withdrawing groups at para-position in aniline were studied at the density functional wB97X-D/6-31G** level. The rotational barrier was found to correlate strongly with shortening of the C–NH2 bond, increase of flattening of NH2 group, decrease in negative natural charge on amino nitrogen, increase in minimum ionization potential around lone pair of amino nitrogen, increase in maximum (positive) electrostatic potential on amino hydrogens, increase in NH2 stretching frequencies, and increase in stabilization energy. The rotational barrier was also found to correlate well with empirical pKa and Hammett σp constants. The rotational barrier is shown to be a reliable quantum mechanical approach to measure p-conjugation in para-substituted anilines. Based on RB a quantitative scale is constructed for the ability of electron-withdrawing substituents to resonate with aniline. A quinone-like structure has been proposed for stronger electron-withdrawing substituents where an extension of resonance stabilization requires the simultaneous presence of electron donor (NH2) and electron-withdrawing groups.
Full Text:
PDFReferences
- L. P. Hammett, The effect of structure upon the reactions of organic compounds. Benzene derivatives, Journal of the American Chemical Society, 1937, 59(1), 96-103.
- T. M. Krygowski, B. T. Stȩpień, Sigma-and pi-electron delocalization: Focus on substituent effects, Chemical Reviews, 2005, 105(10), 3482-3512.
- K. C. Gross, P. G. Seybold, Z. Peralta-Inga, J. S. Murray, P. Politzer, Comparison of quantum chemical parameters and Hammett constants in correlating pKa values of substituted anilines, The Journal of Organic Chemistry, 2001, 66(21), 6919-6925.
- K. C. Gross, P. G. Seybold, Substituent effects on the physical properties and pKa of aniline, International Journal of Quantum Chemistry, 2000, 80(4‐5), 1107-1115.
- T. H. Lowry, K. S. Richardson, Mechanism and theory in organic chemistry (3rd ed.); Harper Collins Publishers: New York, 1987, pp. 232-244.
- A. R. Katritzky, R. D. Topsom. Infrared intensities: A guide to intramolecular interactions in conjugated systems, Chemical Reviews, 1977, 77(5), 639-658.
- T. M. Krygowski, B. T. Stȩpień, Sigma-and pi-electron delocalization: Focus on substituent effects. Chemical Reviews, 2005, 105(10),
-3512.
- P. Ertl, Simple quantum chemical parameters as an alternative to the Hammett sigma constants in QSAR studies, Quantitative Structure‐Activity Relationships, 1997, 16(5), 377-382.
- C. Hansch, A. Leo, R. W. Taft, A survey of Hammett substituent constants and resonance and field parameters, Chemical Reviews, 1991, 91(2), 165-195.
- H. H. Jaffé, A reexamination of the Hammett equation, Chemical Reviews, 1953, 53(2), 191-261.
- G. S. Remya, C. H. Suresh, Quantification and classification of substituent effects in organic chemistry: A theoretical molecular electrostatic potential study, Physical Chemistry Chemical Physics, 2016, 18(30), 20615-20626.
- P. Politzer, J. S. Murray, F. A. Bulat, Average local ionization energy: a review, Journal of Molecular Modeling, 2010, 16(11), 1731-1742.
- H. Szatylowicz, T. Siodla, O. A. Stasyuk, T. M. Krygowski, Towards physical interpretation of substituent effects: the case of meta-and para-substituted anilines, Physical Chemistry Chemical Physics, 2016, 18(17), 11711-11721.
- T. M. Krygowski, N. Sadlej-Sosnowska, Towards physical interpretation of Hammett constants: charge transferred between active regions of substituents and a functional group, Structural Chemistry, 2011, 22(1), 17-22.
- T. M. Krygowski, Crystallographic studies of inter-and intramolecular interactions reflected in aromatic character of π-electron systems, Journal of chemical information and computer sciences, 1993, 33(1), 70-78.
- P. V. Schleyer, M. Manoharan, Z. X. Wang, B. Kiran, H. Jiao, R. Puchta , N. J. van Eikema Hommes, Dissected nucleus-independent chemical shift analysis of π-aromaticity and antiaromaticity, Organic Letters, 2001, 3(16), 2465-2468.
- O. Exner, S. Böhm, Conjugation of two functional groups through an unsaturated system, Journal of Physical Organic Chemistry, 2006, 19(1), 1-9.
- A. N. Rashid, Basis set effects on the ground and excited state of nitrogen containing organic molecules. p-Nitroaniline as a case study, Journal of Molecular Structure: THEOCHEM, 2004, 681(1-3), 57-63.
- L. J. He, J. Chen, F. Q. Bai, R. Jia, J. Wang, H. X. Zhang, Fine-tuning π-spacer for high efficiency performance DSSC: A theoretical exploration with D− π− A based organic dye, Dyes and Pigments, 2017, 141, 251-261.
- X. M. Liu, X. Y. Jin, Z. X. Zhang, J. Wang, F. Q. Bai, Theoretical study on the reaction mechanism of the thermal cis–trans isomerization of fluorine-substituted azobenzene derivatives, RSC advances, 2018, 8(21), 11580-11588.
- W. Zierkiewicz, D. Michalska, P. Hobza, The barrier to internal rotation and electronic effects in para-halogenophenols: theoretical study, Chemical Physics Letters, 2004, 386(1-3), 95-100.
- P. C. Chen and S. C. Chen, Theoretical study of the internal rotational barriers in nitrotoluenes, nitrophenols, and nitroanilines, Computers & Chemistry, 2002, 26(2), 171-178.
- A. Haloui and Y. Arfaoui, A DFT study of the conformational behavior of para-substituted acetophenones in vacuum and in various solvents, Journal of Molecular Structure: THEOCHEM, 2010, 950(1-3), 13-19.
- T. Drakenberg, J. Sommer, R. Jost, The torsional barrier in aromatic carbonyl compounds, Journal of the Chemical Society, Perkin Transactions 2, 1980, (2), 363-269.
- P. V. Bijina, C. H. Suresh, Molecular electrostatic potential analysis of non-covalent complexes, Journal of Chemical Sciences, 2016, 128(10), 1677-1686.
- N. Mohan, C. H. Suresh, A molecular electrostatic potential analysis of hydrogen, halogen, and dihydrogen bonds, The Journal of Physical Chemistry A, 2014, 118(9), 1697-1705.
- A. Albert, E. P. Serjeant, Ionization Constants of Acids and Bases; Methuen: London, 1962.
- D. D. Perrin, Dissociation Constants of Organic Bases in Aqueous Solution; Butterworths: London, 1965.
- C. Hansch, A. Leo, R. W. Taft, A survey of Hammett substituent constants and resonance and field parameters, Chemical Reviews, 1991, 91(2), 165-195.
- SPARTAN’14. Irvine, CA, USA: Wavefunction, 2014.
- J. Donohue, K. Trueblood, The crystal structure of p-nitroaniline, Acta Crystallographica, 1956, 9(11), 960-965.
- N. I. Sadova, N. P. Penionzhkevich, L. V. Vilkov, Study of the structure of the molecule of para-nitroaniline p−C6H4(NO2)(NH2) by gaseous electron diffraction, Journal of Structural Chemistry, 1976, 17(6), 954-956.
- P. J. Krueger, The vibrational mechanism of the fundamental NH2 stretching vibrations in anilines, Canadian Journal of Chemistry, 1962, 40(12), 2300-2316.
- A. F. Lago, J. Z. Davalos, AN de Brito, A density functional and ab initio investigation of the p-aminobenzoic acid molecule, Chemical Physics Letters, 2007, 443(4-6), 232-236.
- T. F. Lai, R. E. Marsh, The crystal structure of p-aminobenzoic acid, Acta Crystallographica, 1967, 22(6), 885-893.
- S. T. Merlino, F. Sartori, The structures of m-cyanoaniline and p-cyanoaniline, Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry, 1982, 38(5), 1476-1480.
- G. Delgado, A. J. Mora, Crystal structure determination of p-bromoaniline using laboratory X-ray powder diffraction data, Materials Science Forum. Transtec Publications, 2001, 378(2),
-797.
- G. Ploug-Sørenson, E. K. Andersen, Structure determination of p-chloroaniline hydrochloride, C6H7ClN+.Cl−, and redetermination of p-chloroaniline, C6H6ClN, Acta Crystallographica Section C: Crystal Structure Communications, 1985, 41(4), 613-615.
- D. G. Lister, J. K. Tyler, J. H. Høg, N. W. Larsen, The microwave spectrum, structure and dipole moment of aniline, Journal of Molecular Structure, 1974, 23(2), 253-264.
- G. Schultz, G. Portalone, F. Ramondo, A. Domenicano, I. Hargittai, Molecular structure of aniline in the gaseous phase: A concerted study by electron diffraction and ab initio molecular orbital calculations, Structural Chemistry, 1996, 7(1), 59-71.
- G. Roussy, A. Nonat, Determination of the equilibrium molecular structure of inverting molecules by microwave spectroscopy: application to aniline, Journal of Molecular Spectroscopy, 1986, 118(1), 180-188.
- M. A. Palafox, M. Gill, N. J. Nunez, V. K. Rastogi, L. Mittal, R. Sharma, Scaling factors for the prediction of vibrational spectra. II. The aniline molecule and several derivatives, International journal of quantum chemistry, 2005, 103(4), 394-421.
- A. Hastie, D. G. Lister, R. L. McNeil, J. K. Tyler, Substituent effects in benzene: the microwave spectrum of p-fluoroaniline, Journal of the Chemical Society D: Chemical Communications, 1970, (2), 108-109.
- Larsen NW, Hansen EL, Nicolaisen FM. Far infrared investigation of aniline and 4-fluoroaniline in the vapour phase. Inversion and torsion of the amino group, Chemical Physics Letters, 1976, 43(3), 584-586.
- E. Klein, V. Lukeš, Z. Cibulková, J. Polovková, Study of N–H, O–H, and S–H bond dissociation enthalpies and ionization potentials of substituted anilines, phenols, and thiophenols, Journal of Molecular Structure: THEOCHEM, 2006, 758(2-3), 149-159.
- G. Fronza, R. Mondelli, F. Lelj, E. W. Randall, C. A. Veracini, Structural changes induced in the NH2 group of aniline by substituents: The structure and orientation of para-bromo-and para-nitroaniline [1-15N] in nematic liquid crystal solvents by NMR, Journal of Magnetic Resonance (1969), 1980, 37(2), 275-284.
- S. F. Mason, 723. The frequencies and intensities of the N–H stretching vibrations in primary amines, Journal of the Chemical Society (Resumed), 1958, 3619-3627.
- M. Haeberlein, J. S. Murray, T. Brinck, P. Politzer, Calculated electrostatic potentials and local surface ionization energies of para-substituted anilines as measures of substituent effects, Canadian Journal of Chemistry, 1992, 70(8), 2209-2214.
- P. J. Krueger, H. W. Thompson, Vibrational band intensities in substituted anilines, Proceedings of the Royal Society of London. Series A. Mathematical and Physical Sciences, 1957, 243(1233), 143-153.
- D. V. Kumar, V. A. Babu, G. R. Rao, G. C. Pandey, Vibrational analysis of substituted anilines, anisoles and anisidines: Part I. Vibrational spectra and normal coordinate analysis of some nitro compounds, Vibrational Spectroscopy, 1992, 4(1), 39-57.
- A. H. Yateem, Rotational Barrier and Conjugation: Theoretical Study of Resonance Stabilization of Various Substituents for the Donors NH2 and OCH3 in Substituted 1, 3-Butadienes, Indonesian Journal of Chemistry, 2019, 19(4), 1055-1065.
DOI: http://dx.doi.org/10.13171/mjc02004161378ahy
Refbacks
- There are currently no refbacks.
Copyright (c) 2020 Mediterranean Journal of Chemistry
