Electrochemical study and characterization of tin coatings with and without glucose-based additive
Abstract
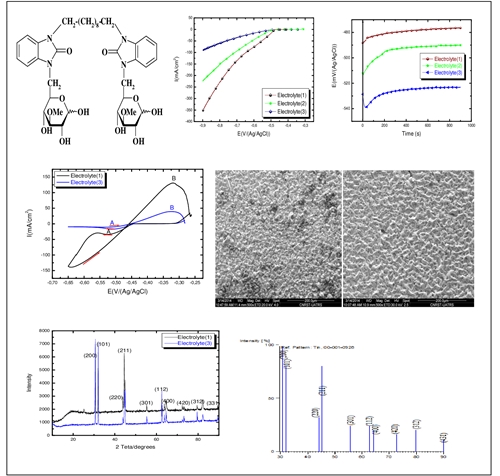
The tin coating was elaborated electrolytically on an ordinary steel substrate in SnSO4 based electrolyte in acid medium with additive (bis-glycobenzimidazolone) at ambient temperature. The pH is maintained at 1.2±0.2 Bis-glycobenzimidazolone influence on the electrochemical properties of the tin coating was investigated using stationary polarization, chronopotentiometry, and cyclic voltammetry techniques. These studies show an apparent decrease in cathodic peak current and a drop in potential. The deposition rate also decreases as the concentration of the additive increases. SEM (Scanning Electron Microscopy) observation and XRD (X-ray Diffraction) analysis showed that the coating consists of good surface quality of the deposit elaborated by the addition of an optimal concentration of bis-glycobenzimidazolone (10-3M) in the electrolyte, which constitutes the continuation of a preliminary study.
Full Text:
PDFReferences
- Y. Salhi, S. Cherrouf, J. Tellal, M. Cherkaoui, Electrodeposition of Cu-Zn-Sn coating in citrate medium, Mediterr. J. Chem., 2020, 9(6), 456-467.
- M. Charrouf, S. Bakkali, M. Cherkaoui, M. EL Amrani, Influence of decyl glucoside on the electrodeposition of tin J. Serbian. Chem., 2006, 71, 659-666. ,
- V. W. Zhang, J. Guebey, M. Toben, K. Weitershaus, Luzern/Schweiz., 2011, 520-528.
- E. Chason, N. Jadhav, F. Pei, E. Buchovecky, A. Bower, Growth of whiskers from Sn surfaces: Driving forces and growth mechanisms, Progress in Surf. Sci., 2013, 88, 103-131.
- K. Chat-Wilk, E. Rudnik, G. Włoch, Importance of anions in the electrodeposition of nickel from gluconate solutions, Ionics, 2021, 27, 4393–4408.
- S. Bakkali, T. Jazouli, M. Cherkaoui, M. Ebn Touhami, N. EL Hajjaji, E. Chassaing, Influence of M12 organic additive on the electrodeposition of tin from an acid sulfate solution, Plat. and Surf. Finish., 2003 ,90, 1,
-49.
- K. N. Strafford, A. Reed, Coatings reduce the fouling of
microfiltration membranes, Coat. & Surf. Treat. for Corr. and Wear Resist., 1984, 74.
- G. S. Tzeng, S. H. Lin, Y. Y. Wang, C. C. Wan, Effects of additives on the electrodeposition of tin from an acidic Sn (II) bath, J. Electroanal. Chem., 1996, 26, 419-423.
- E. Guaus, J. Torrent-Burgues, Tin–zinc electrodeposition from sulphate–gluconate baths, Journal of Electroanalytical Chemistry, 2003, 549, 25-36.
- A. Aragon, M. G. Figueroa, R. E. Gana, J. H. Zagal, Effect of a polyethoxylate surfactant on the electrodeposition of tin, J. Appl. Electrochem., 1992, 22, 558 -562.
- G. S. Thzeng, Plat. and Surf. Finish., 1995 ,82,67-72.
- S. Meibuhr, E. Yeager, A. Kozawa, F. Hovorka, The electrochemistry of tin: I. Effects of nonionic addition agents on electrodeposition from stannous sulfate solutions, J. Electrochem. Soc., 1963, 110, 190-202.
- C. L. Rinne, J. J. Hren, P. S. Fedkiw, Electrodeposition of tin needle-like structures, J. Electrochem. Soc. 2002,149, C150-158.
- E. Rudnik, G. Chowaniec, Effect of organic additives on electrodeposition of tin from acid sulfate solution, Metallurgy and Foundry Engineering, 2018, 44(1), 41-52.
- I. Yawata, S. Company, UK Patent, 1966, 16-21.
- Zavarine, I. Khaselev, O. Y. Zhang, J. Electrochem. Soc. 2003 ,150,202-207.
- P. A. Kohl, The high-speed electrodeposition of Sn/Pb alloys, J. Electrochem. Soc., 1982, 129, 1196-1201.
- C.T.J. Low, F.C. Walsh, The influence of a perfluorinated cationic surfactant on the electrodeposition of tin from a methanesulfonic acid bath, J. Electroanal. Chem, 2008, 615,
-102.
- B. Lakhrissi, B. El Azzaoui, A. Nabil, E. M. Essassi, M. Massoui, G. Goethals, P. Villa, C. Solans, N. Azemar, F. Comelles, M. J. Garcia-Celma, N. Sadurni, P. G. Jorn, Com. Esp. Deterg., 2000, 30, 233–243.
- S. Cherrouf, Y. Salhi, A. Benabida, H. Elgrini, M. Cherkaoui, L. Lakhrissi, B.Lakhrissi, Elaboration of tin deposits in the presence of Bis-glucobenzimidazolone, Moroccan Journal of Chemistry, 2014, 2(3), 175-180.
- L. H. Mendoza-Huizar, J. Robles, M. Palomar-Pardavé, Nucleation and growth of cobalt onto different substrates: Part II. The upd-opd transition onto a gold electrode, Journal of Electroanalytical Chemistry, 2003, 545, 39-45.
- M. A.Shikun, O. N. Vrublevskaya, T. N. Vorobyova, Functions of 2-butyne-1, 4-diol in the process of tin-silver alloy electrodeposition from the acidic sulfate solution, Surfaces and Interfaces, 2021, 24, 101059.
- S. Cherrouf, Y. Salhi, M. Cherkaoui, B. Lakhrissi, N. Lakhrissi, Study of the influence of a bis-glycobenzimidazolone on the reduction of stannous ions, J. Mater. Environ., 2016, 7(7), 2595-2602.
- S. Bakkali, R. Touir, M. Cherkaoui, M. Ebn Touhami, Influence of S-dodecyl-mercapto benzimidazole as an organic additive on electrodeposition of tin, Surface & Coatings Technology, 2014, S0257(14),
-8991.
- Joint Committee on Powder Diffraction Standards (JCPDS) International Center for Diffraction File PDF-2 Database 2000, 1-49.
DOI: http://dx.doi.org/10.13171/mjc02110281589salhi
Refbacks
- There are currently no refbacks.
Copyright (c) 2021 Mediterranean Journal of Chemistry
