Synthesis, X-ray structure and antibacterial evaluation of P-[{[bis (dimethylamino)phosphoryl]amino]} (2-chloroquinolin-3-yl) methyl]-, P- (quinolin-3-yl) tetramethylphosphonic diamides
Abstract
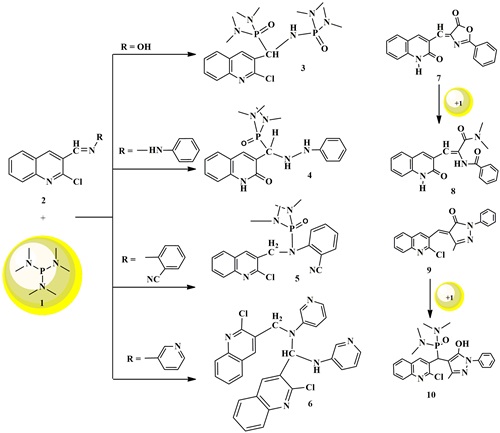
A new P-bis [(dimethylamino) phosphoryl] amino} (2-chloroquinolin-3-yl) methyl]- N, N,N',N'- tetramethylphos-phonic diamide was synthesized by the reaction of trisdiaminophosphine to 2-chloroquinolin-3-aldoxime. The structure of P-bis [(dimethylamino) phosphoryl] amino} (2-chloroquinolin-3-yl) methyl]- N, N,N',N'- tetramethyl-phosphonic diamide is confirmed by X-ray diffraction studies. On the other hand, the neucluphilic attack of aminophosphine on 2-chloroquinolin-3-carboimines produced different products, depending on the stability of dipolar phosphorylhexamethyl amide intermediates. Furthermore, the reaction of 3-((5-oxo-2-phenyl-oxazol-4(5H)-ylidene) methyl) quinolin-2(1H)-one with phosphine yielded alkene adduct, the Z structure is confirmed by X-ray analysis. P-[(2-Chloro-quinolin-3-yl)(5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl) methyl]-N,N,N,N,tetramethyl-phosphonic diamide was obtained from reaction of 4-( (2-chloro-quinolin-3-yl) methylene)-3-methyl-1- phenyl-1H-pyrazol-5(4H)- one with reagent trisdiaminophosphine. Antibacterial evaluation of most compounds exhibited moderate activity to Gram positive and Gram-negative bacteria species.
Full Text:
PDFReferences
- H. U. Okoroiwu, I. A. Iwara, Dichlorvos toxicity: a public health perspective, Interdiscip Toxicol, 2018, 11, 129–37.
- W. Andrew, Pharmaceutical Manufacturing Encyclopedia, 3rd ed, Norwish NY: Elsevier, 2007, 1, 3846.
- Q. Yao, L. Reng, M. Ran, J. He, D. Xiang, Review on the structures of phosphorus-containing drugs used in clinical practice, Medicine in drug discovery, 2019, 41, 139–46.
- J. B. Rodriguez, C. Gallo-Rodriguez, The role of the phosphorus atom in drug design, Chem. Med. Chem., 2019, 14,190–126.
- Y. Hanxiao, H. Yang, S. Enxue, Tang Wenjun, Development and clinical application of phosphorus-containing drugs, Medicine in Drug Discovery, 2020, 8, 100063.
- S. Pol, M. Corouge, A. Vallet-Pichard, Daclatasvir- sofosbuvir combination therapy with or without ribavirin for hepatitis C virus infection: from the clinical trials to real life, Medical Devices: Evidence and Research, 2016, 8, 21-26 .
- M. Agostini, E. Andres, A. Sims, R. Graham, T. Sheahan, X. Lu, Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease, American Society Microbiology, 2018, 9, e00221-18.
- J. S. Dhau, A. Singh, A. Singh, B. S. Sooch, A Study on the Antioxidant Activity of Pyridylselenium Compounds and their Slow Release from Poly(acrylamide) Hydrogels, Phosphorus, Sulfur, and Silicon and the Related Elements, 2014, 189, 687–699.
- A. Singh, A. Kaushik, Ja. S. Dhau, R. Kumar, Exploring coordination preferences and biological applications of pyridyl-based organochalcogen (Se, Te) ligands, Coordination Chemistry Reviews, 2022, 450, 214254.
- M. Hesse, H. Meier, B. Zeeh, Spektroskopische Methoden in der Organishen Chemic, Georg Thieme Verlag, Stuttgard, 1991.
- M. Arsanious, Reactions of Trisdialkylaminophosphines with Acrylohydrazide and Imidazolinone Derivatives for the Synthesis of New Organophosphorus Derivatives, Synthetic Communications, 2009, 39, 1626-1639.
- M. Arsanious, S. S. Maigali, Isatin Imines in the Reaction with Iminophosphine: X-Ray Structure of Phospholane Derivatives, Synthetic Communications, 2014, 44, 202-214.
- E. S. Batyeva, V. A. Al' fonsov, M. Z. Kaufman, A. N. Pudovik, Isomerization of β-propiolactone in the presence of amides of phosphorus acids, Russian Chemical Bulletin, 1976, 25,1166.
- A. Lausi, M. Polentarutti, S. Onesti, J. Plaisier, E. Busetto, G. Bais, L. Barba, A. Cassetta, G. Campi, D. Lamba, A. Pifferi, S. Mande, D. Sarma, S. Sharma, G. Paolucci, Status of the crystallography beamlines at Elettra, European Physical Journal Plus, 2015,43, 1-8.
- W. Kabsch, XDS, Acta Crystallography Sect. D, 2010, 66, 125-132.
- G. Sheldrick, SHELXT-Integrated Space-Group and Crystal-Structure Determination, Acta Crystallographica Sect., 2015, A71, 3-8.
- C. Macrae, J. Bruno, J. Chisholm, P. Edgington, P. McCabe, E. Pidcock, L. Rodriguez- Monge, R. Taylor, J van de Streek, P. Wood, new features for the visualization and investigation of crystal structures, Journal of Applied Crystallography, 2008, 41, 466-470.
- M. Arsanious, S. Darwish, El-S Shalaby, D. El-Ghwas, Synthesis, X-ray, DFT studies and antimicrobial properties of new quinolinylphosphonates, Letters in Organic Chemistry, 2019, 8, 668.
- J. Son, S. Tamang, J. Hoefelmeyer, Crystal structure of bis(3-bromomesityl)(quinolin-1-ium-8-yl)boron(III) tribromide, Acta Crystallographica Sect. E, 2015, 71, 1114-1116.
- S. J. Tu, Y. Zhang, R. H. Jia, 13-(4-Fluoro¬phen¬yl)-12H-benzo[f]indeno[1,2-b]quinolin-12-one, Acta Crystallographica Sect. E, 2006, 62, o3930- o3931.
- A. Asiri, A. Al-Youbi, H. Faidallah, SW Ng, 2-Amino-4-(4-chloro¬phen¬yl)-5,6-di¬hydro¬benzo [h] quinoline-3-carbo¬nitrile–3-amino-1-(4-chloro-phen¬yl)-9,10-di¬hydro¬phenanthrene-2,4-dicarbo-nitrile (1/ 4), Acta Crystallographica Sect. E, 2011, 67, o2873-o2874.
- S. J. Tu, Y. Zhang, R. H. Jia, 7-(4-Fluoro¬phen¬yl)-8H-benzo[h]indeno[1,2-b]quinolin-8-one, Acta Crystallographica Sect. E, 2006, 62, o3928- o3929.
- A. Rajapakse, R. Hillebrand, S. Lewis, Z. Parsons, C. Barnes, K. Gates, Crystal structure of N-(quinolin-6-yl) hydroxyl¬amine, Acta Crystallographica Sect. E, 2014, 70, 322-324.
- I. Gama, M. Souza, J. Wardell, E. Tiekink,7-Chloro-4-(2-hy¬droxy¬ethyl¬amino)¬quinolin-1-ium chloride E, Acta Crystallographica Sect. E,
, 70, o385- o386.
- A. Singh, S. N.Maximoff, P. Brandão, J. S.Dhau, Crystal structures of bis (2-methoxy-3-pyridyl) diselenide and bis (2-methoxy-3-pyridyl) ditelluride: an investigation by X-ray crystallography and DFT calculations, Journal of Molecular Structure, 2021, 1240, 130568.
- J. S. Dhau, A. Singh, A. Singh, B. S. Sooch, P. Brandão, V. Félix, Synthesis and antibacterial activity of pyridylselenium compounds: Self-assembly of bis(3-bromo-2-pyridyl)diselenide via intermolecular secondary and π⋯π stacking interactions, Journal of Organometallic Chemistry, 2014, 766, 57-66.
- D. N. Muanza, B. W. Kim, K. L. Euler, L. Williams, Antibacterial and Antifungal Activities of Nine Medicinal Plants from Zaire, International Journal Pharmacognosy, 1994, 32, 337-345.
- O. N. Irobi, M. Moo-Young, W., Anderson, Synthesis and Biological Evaluation of Novel 6-(3-(4,5-Dihydro-1,5-diphenyl-1H-pyrazol-3-yl)phenylamino) Pyridazin-3(2H)-one Derivatives, International Journal Pharmacognosy, 1996, 34, 87-90.
- A. H. Kategaonkar, R. U. Pokalwar, S. S. Sonar, V. U. Gawali, M. S. Shingare Synthesis, in vitro antibacterial and antifungal evaluations of new α-hydroxyphosphonate and new α-acetoxyphosphonate derivatives of tetrazolo [1, 5-a] quinoline, European Journal of Medicinal Chemistry, 2010, 45, 1128-1132.
DOI: http://dx.doi.org/10.13171/mjc02206211632arsanious
Refbacks
- There are currently no refbacks.
Copyright (c) 2022 Mediterranean Journal of Chemistry
