Elimination of Terbinafine Hydrochloride Antifungal Drug Traces from Water, Pharmaceutical Formulations and Blood Plasma using Low-Cost Bio and Synthetic Sorbents
Abstract
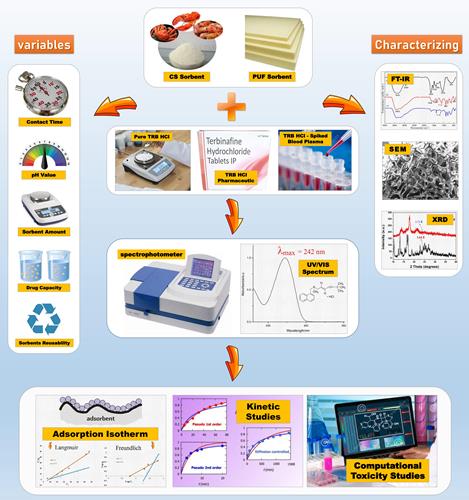
: Chitosan (CS) biosorbent and polyurethane foam (PUF) synthetic sorbent have been utilized to eliminate terbinafine hydrochloride (TRB HCl) antifungal drug in its pure and pharmaceutical forms from both contaminated aqueous and biological media using a batch process. The experimental conditions for efficient removal of TRB HCl for both CS and PUF were optimized depending on various experimental parameters such as the pH of the solution, contact periods, initial TRB HCl concentration, and sorbents dosage in the solution.
SEM, FT-IR, and XRD characterizations were carried out to study the adsorption of a drug by both sorbents. The optimum conditions for removing TRB HCl by CS and PUF were achieved at a pH of 8.5 and a contact time of 60 min at 250 rpm, using 0.4 g for both sorbents. The measured spectrophotometric absorbance at λmax of TRB HCl was 242 nm. In addition, the zero-point charge (pHpzc) was determined for the studied sorbents. The pHpzc of the surface of sorbents has shown that electrostatic attraction is one of the mechanisms in TRB HCl sorption. The adsorption process was modeled using the pseudo-first-order, pseudo-second-order, Elovich, and intraparticle diffusion kinetic models. The results indicated that the adsorption of TRB HCl on CS and PUF does follow a pseudo-first-order type of reaction kinetics. The adsorption process was modeled using Langmuir and Freundlich isotherms. The adsorption data found that the Freundlich isotherm model was more suitable for the PUF sorbent, while the Langmuir isotherm model better fit the CS biosorbent.
Evaluation of the experimental data using the Langmuir equation revealed that the maximum adsorption capacities of PUF and CS were 2.807 and 1.2297 mg. g-1, respectively. The solution was also used to estimate TRB HCl in its pharmaceutical form, and the assessed recoveries were 97.25 and 98.437% for CS and PUF, respectively. The proposed procedure was validated for other complex mediums by removing TRB HCl from spiked human blood plasma. In-silico aquatic toxicity forecast of TRB HCl was also carried out.
Full Text:
PDFReferences
- G. Crini, E. Lichtfouse, Wastewater treatment: an overview, Green adsorbents for pollutant removal, 2018, 1-21.
- P. M. Pakdel, S. J. Peighambardoust, Review on recent progress in chitosan-based hydrogels for wastewater treatment application, Carbohydrate Polymers, 2018, 201, 264-279.
- S. Mudgal, A. De Toni, S. Lockwood, K. Salès, T. Backhaus, B. H. Sorensen, Study on the environmental risks of medicinal products: Final Report prepared by BIO Intelligent Service, Executive Agency for Health and Consumers, 2013.
- M. Sousa, C. Gonçalves, V. J. Vilar, R. A. Boaventura, M. Alpendurada, Suspended TiO2-assisted photocatalytic degradation of emerging contaminants in a municipal WWTP effluent using a solar pilot plant with CPCs, Chemical Engineering Journal, 2012, 198, 301-309.
- M. Patel, R. Kumar, K. Kishor, T. Mlsna, C. U. Pittman Jr, D. Mohan, Pharmaceuticals of emerging concern in aquatic systems: chemistry, occurrence, effects, and removal methods, Chemical Reviews, 2019, 119, 3510-3673.
- R. Mrutyunjayarao, S. Naresh, K. Pendem, P. R. Rao, C. Sasttry, U. V. Prasad, Three simple spectrometric determination of terbinafine hydrochloride (TRB) in pure state and tablets, Proceedings of the National Academy of Sciences, India Section A: Physical Sciences, 2012, 82, 221-224.
- B. Kanakapura, V. K. Penmatsa, Analytical methods for determination of terbinafine hydrochloride in pharmaceuticals and biological materials, Journal of Pharmaceutical Analysis, 2016, 6, 137-49.
- British pharmacopoeia, https://www.pharmacopoeia.com. 2016, 15 Sept 2020.
- J. Fick, H. Söderström, R. H. Lindberg, C. Phan, M. Tysklind, D. J. Larsson, Contamination of surface, ground, and drinking water from pharmaceutical production, Environmental Toxicology and Chemistry, 2009, 28, 2522-2527.
- S. G. Cardoso, E. E. Schapoval, UV spectrophotometry and nonaqueous determination of terbinafine hydrochloride in dosage forms, Journal of AOAC International, 1999, 82, 830-833.
- K. Patel, V. Karkhanis, A validated HPTLC method for determination of Terbinafine hydrochloride in pharmaceutical solid dosage form, International Journal of Pharmaceutical Sciences, 2012, 3, 4492-4495.
- J. Denouël, H. Keller, P. Schaub, C. Delaborde, H. Humbert, Determination of terbinafine and its desmethyl metabolite in human plasma by high-performance liquid chromatography, Journal of Chromatography B: Biomedical Sciences Applications, 1995, 663, 353-359.
- C. Wang, Y. Mao, D. Wang, G. Yang, Q. Qu, X. Hu, Voltammetric determination of terbinafine in biological fluid at glassy carbon electrode modified by cysteic acid/carbon nanotubes composite film, Bioelectrochemistry, 2008, 72, 107-115.
- F. Faridbod, M. R. Ganjali, P. Norouzi, Potentiometric PVC membrane sensor for the determination of terbinafine, International Journal of Electrochemical Science, 2013, 8, 6107-6117.
- M. Chennaiah, T. Veeraiah, T. V. Kumar, G. Venkateshwarlu, Extractive spectrophotometric methods for determination of terbinafine hydrochloride in pharmaceutical formulations using some acidic triphenylmethane dyes, Indian Journal of Chemical Technology, 2012, 19, 218-221.
- F. Belal, M. S. El-Din, M. Eid, R. El-Gamal, Spectrofluorimetric determination of terbinafine hydrochloride and linezolid in their dosage forms and human plasma, Journal of fluorescence, 2013, 23, 1077-1087.
- K. Mielech-Łukasiewicz, A. Dąbrowska, Comparison of boron-doped diamond and glassy carbon electrodes for determination of terbinafine in pharmaceuticals using differential pulse and square wave voltammetry, Analytical Letters, 2014, 47, 1697-1711.
- F. S. Felix, L. M. Ferreira, P. d. O. Rossini, C. L. do Lago, L. Angnes, Quantification of terbinafine in pharmaceutical tablets using capillary electrophoresis with contactless conductivity detection and batch injection analysis with amperometric detection, Talanta, 2012, 101, 220-225.
- N. Brignol, R. Bakhtiar, L. Dou, T. Majumdar, F. Tse, Quantitative analysis of terbinafine (Lamisil®) in human and minipig plasma by liquid chromatography-tandem mass spectrometry, Rapid communications in mass spectrometry, 2000, 14, 141-149.
- R. Choumane, S. Peulon, Electrodeposited birnessite thin film: An efficient eco-friendly sorbent for removing heavy metals from water, Colloids Surfaces A: Physicochemical Engineering Aspects, 2019, 577, 594-603.
- A. M. Elgarahy, A. Akhdhar, A. S. Al-Bogami, K. Z. Elwakeel, Magnetically separable solid phase extractor for static anionic dyes adsorption from aqueous solutions, Surfaces and Interfaces, 2022, 30, 101962.
- A. A. Yakout, M. A. Shaker, K. Z. Elwakeel, W. Alshitari, Response surface methodological optimization of batch Cu (II) sorption onto succinic acid functionalized SiO2 nanoparticles, Canadian Journal of Chemistry, 2019, 97, 277-286.
- A. A. Yakout, M. A. Shaker, K. Z. Elwakeel, W. Alshitari, Lauryl sulfate@ magnetic graphene oxide nanosorbent for fast methylene blue recovery from aqueous solutions, Journal of Dispersion Science and Technology, 2019, 40, 707-715.
- S. Kanamarlapudi, V. K. Chintalpudi, S. Muddada, Application of biosorption for removal of heavy metals from wastewater, Biosorption, 2018, 18, 69.
- A. Elgarahy, K. Elwakeel, S. Mohammad, G. Elshoubaky, A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process, Cleaner Engineering and Technology, 2021, 4, 100209.
- D. Huang, B. Li, J. Ou, W. Xue, J. Li, Z. Li, T. Li, S. Chen, R. Deng, X. Guo, Megamerger of biosorbents and catalytic technologies for the removal of heavy metals from wastewater: Preparation, final disposal, mechanism and influencing factors, Journal of Environmental Management, 2020, 261, 109879.
- S. Islam, M. A. R. Bhuiyan, M. N. Islam, Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering, Journal of Polymers and the Environment, 2016, 25, 854-866.
- T. Chang, Cell encapsulation technology and therapeutics, Springer Science & Business Media, 2013.
- M. S. Almughamisi, Z. A. Khan, W. Alshitari, K. Z. Elwakeel, Recovery of chromium (VI) oxyanions from aqueous solution using Cu (OH) 2 and CuO embedded chitosan adsorbents, Journal of Polymers and the Environment, 2020, 28, 47-60.
- K. Z. Elwakeel, A. S. Al-Bogami, A. M. Elgarahy, Efficient retention of chromate from industrial wastewater onto a green magnetic polymer based on shrimp peels, Journal of Polymers and the Environment, 2018, 26.
- S. Wei, Y. C. Ching and C. H. Chuah, Synthesis of chitosan aerogels as promising carriers for drug delivery: A review, Carbohydrate Polymers, 2019, 231, 115744.
- M. Saraji, M. Tarami, N. Mehrafza, Preparation of a nano-biocomposite film based on halloysite-chitosan as the sorbent for thin film microextraction, Microchemical Journal, 2019, 150, 104171.
- T. Zidan, A. E. Abdelhamid, E. Zaki, N-Aminorhodanine modified chitosan hydrogel for antibacterial and copper ions removal from aqueous solutions, International Journal of Biological Macromolecules, 2020, 158, 32-42.
- K. Z. Elwakeel, Environmental application of chitosan resins for the treatment of water and wastewater: a review, Journal of dispersion science and technology, 2010, 31, 273-288.
- V. Lemos, M. Santos, E. Santos, M. Santos, W. Dos Santos, A. Souza, D. De Jesus, C. Das Virgens, M. Carvalho, N. Oleszczuk, Application of polyurethane foam as a sorbent for trace metal pre-concentration—A review, Spectrochimica acta part B: Atomic spectroscopy, 2007, 62, 4-12.
- M. S. El-Shahawi, H. Alwael, A novel platform based on gold nanoparticles chemically impregnated polyurethane foam sorbent coupled ion chromatography for selective separation and trace determination of phosphate ions in water, Microchemical Journal, 2019, 149, 103987.
- H. Li, L. Liu, F. Yang, Hydrophobic modification of polyurethane foam for oil spill cleanup, Marine Pollution Bulletin, 2012, 64, 1648-1653.
- E. Moawed, N. Burham, M. El-Shahat, Separation and determination of iron and manganese in water using polyhydroxyl polyurethane foam, Journal of the Association of Arab Universities for Basic and Applied Sciences, 2013, 14, 60-66.
- M. F. Silva, D. Rigo, V. Mossi, R. M. Dallago, P. Henrick, G. de Oliveira Kuhn, C. Dalla Rosa, D. Oliveira, J. V. Oliveira, H. Treichel, Evaluation of enzymatic activity of commercial inulinase from Aspergillus niger immobilized in polyurethane foam, Food and Bioproducts Processing, 2013, 91, 54-59.
- A. A. Nikkhah, H. Zilouei, A. Asadinezhad, A. Keshavarz, Removal of oil from water using polyurethane foam modified with nanoclay, Chemical Engineering Journal, 2015, 262, 278-285.
- A. Keshavarz, H. Zilouei, A. Abdolmaleki, A. Asadinezhad, Enhancing oil removal from water by immobilizing multi-wall carbon nanotubes on the surface of polyurethane foam, Journal of Environmental Management, 2015, 157, 279-286.
- S. Ranote, D. Kumar, S. Kumari, R. Kumar, G. S. Chauhan, V. Joshi, Green synthesis of Moringa oleifera gum-based bifunctional polyurethane foam braced with ash for rapid and efficient dye removal, Chemical Engineering Journal, 2019, 361, 1586-1596.
- S. M. A. Azeem, S. Ali, M. F. El-Shahat, Sorption characteristics of caffeine onto untreated polyurethane foam: application to its determination in human plasma, Analytical Sciences, 2011, 27, 1133-1133.
- K. Patel, V. Karkhanis, A validated HPTLC method for determination of Terbinafine hydrochloride in pharmaceutical solid dosage form, International Journal of Pharmaceutical Sciences and Research, 2012, 3, 4492-4495.
- G. Crini, E. Lichtfouse, L. D. Wilson, N. Morin-Crini, Adsorption-oriented processes using conventional and non-conventional adsorbents for wastewater treatment, Springer, Cham, 2018, 23-71.
- N. Fiol, I. Villaescusa, Determination of sorbent point zero charge: usefulness in sorption studies, Environmental chemistry letters, 2009, 7, 79-84.
- S. Ali, S. M. Sirry, H. A. Hassanin, Removal and characterisation of Pb(II) ions by xylenol orange-loaded chitosan: equilibrium studies, International Journal of Environmental Analytical Chemistry, 2020, 1-13.
- Y. Luo, Q. J. J. o. F. P. Wang, Beverages, Recent advances of chitosan and its derivatives for novel applications in food science, Journal of Food Processing & Beverages, 2013, 1, 1-13.
- R. Gómez-Rojo, L. Alameda, Á. Rodríguez,
V. Calderón, S. Gutiérrez-González, Characterization of polyurethane foam waste for reuse in eco-efficient building materials, Polymers, 2019, 11, 359-374.
- Z. Abdeen, S. G. Mohammad, Study of the adsorption efficiency of an eco-friendly carbohydrate polymer for contaminated aqueous solution by organophosphorus pesticide, Open Journal of Organic Polymer Materials, 2013, 2014.
- L. Jiao, H. Xiao, Q. Wang, J. Sun, Thermal degradation characteristics of rigid polyurethane foam and the volatile products analysis with TG-FTIR-MS, Polymer Degradation and Stability, 2013, 98, 2687-2696.
- S. Kumar, J. Koh, Physiochemical, optical and biological activity of chitosan-chromone derivative for biomedical applications, International Journal of Molecular Sciences, 2012, 13, 6102-6116.
- J. Li, J.-L. Gong, G.-M. Zeng, P. Zhang, B. Song, W.-C. Cao, H.-Y. Liu, S.-Y. Huan, Zirconium-based metal-organic frameworks loaded on polyurethane foam membrane for simultaneous removal of dyes with different charges, Journal of colloid and interface science, 2018, 527,
-279.
- A. Dong, G. Hou, M. Feng, A. Li, Properties of amphoteric polyurethane waterborne dispersions. III. Isoelectric points and precipitation, Journal of Polymer Science Part B: Polymer Physics, 2002, 40, 2440-2448.
- M. M. Ibrahim, W. W. Ngah, M. Norliyana, W. W. Daud, M. Rafatullah, O. Sulaiman, R. Hashim, A novel agricultural waste adsorbent for the removal of lead (II) ions from aqueous solutions, Journal of Hazardous Materials, 2010, 182, 377-385.
- A. Kurniawan, H. Sutiono, N. Indraswati,
S. Ismadji, Removal of basic dyes in binary system by adsorption using rarasaponin–bentonite: Revisited of extended Langmuir model, Chemical Engineering Journal, 2012, 189, 264-274.
- R. A. Al-Bayati, A. S. Ahmed, Adsorption-desorption of trimethoprim antibiotic drug from aqueous solution by two different natural occurring adsorbents, International Journal of Chemistry, 2011, 3, 21-30.
- M. Thanou, J. Verhoef, H. Junginger, Oral drug absorption enhancement by chitosan and its derivatives, Advanced Drug Delivery Reviews, 2001, 52, 117-126.
- Y.-C. Chung, Y.-H. Li, C.-C. Chen, Pollutant removal from aquaculture wastewater using the biopolymer chitosan at different molecular weights, Journal of Environmental Science and Health, Part A: Toxic/Hazardous Substances and Environmental Engineering, 2005, 40,
-1790.
- L. Abbasi, M. Faraji, M. Bahmaie, Extracting trace amount of terbinafine hydrochloride in biological fluids and wastewater samples using solid‐phase‐extraction based on magnetic nanoparticles followed by HPLC‐UV analysis, Asia‐Pacific Journal of Chemical Engineering, 2014, 9, 826-833.
- V. Oskoei, M. Dehghani, S. Nazmara, B. Heibati, M. Asif, I. Tyagi, S. Agarwal, V. K. Gupta, Removal of humic acid from aqueous solution using UV/ZnO nano-photocatalysis and adsorption, Journal of Molecular Liquids, 2016, 213, 374-380.
- A. N. Amro, M. K. Abhary, M. M. Shaikh, S. Ali, Removal of Lead and Cadmium Ions from Aqueous Solution by Adsorption on a Low-Cost Phragmites Biomass, Processes, 2019, 7, 406-417.
- H. K. Hami, R. F. Abbas, A. A. Waheb, M. A. Abed, A. A. Maryoosh, Isotherm and pH Effect Studies of Tetracycline Drug Removal from Aqueous Solution Using Cobalt Oxide Surface, Al-Nahrain Journal of Science, 2019, 22, 12-18.
- F. F. Al-Qaim, Adsorption of Malachite Green (MG) on low cost–adsorbent from aqueous solution, Journal of Babylon University for Pure Applied Sciences, 2011, 1, 48-56.
- S. K. Ghati, Removal of Chlorpyrifos (Dursban) Pesticide from Aqueous Solutions using Barley Husks, Ibn al-Haitham Journal for Pure and Applied Science, 2017, 29, 54-68.
- E. A. Assirey, S. M. Sirry, H. A. Burkani, M. Ibrahim, Biosorption of Zinc (II) and Cadmium (II) Using Ziziphus Spina Stones, Journal of Computational Theoretical Nanoscience, 2018, 15, 3102-3108.
- S. Afroze, T. K. Sen, H. M. Ang, Adsorption removal of zinc (II) from aqueous phase by raw and base modified Eucalyptus sheathiana bark: Kinetics, mechanism and equilibrium study, Process Safety and Environmental Protection, 2016, 102, 336-352.
- Y.-S. Ho and G. McKay, Pseudo-second order model for sorption processes, Process biochemistry, 1999, 34, 451-465.
- A. Taha, E. Da'na, H. A. Hassanin, Modified activated carbon loaded with bio-synthesized Ag/ZnO nanocomposite and its application for the removal of Cr (VI) ions from aqueous solution, Surfaces and Interfaces, 2021, 23, 100928.
- O. Aworanti, S. Agarry, Kinetics, isothermal and thermodynamic modelling studies of hexavalent chromium ions adsorption from simulated wastewater onto Parkia biglobosa-Sawdust derived acid-steam activated carbon, Methods, 2017, 10, 11.
- H. E. I. Ismi, A. Ouass, L. Chafki, H. Essebaai, H. Bousfiha, A. Lebkiri, E. H. Rifi, Kinetic Analysis and Isotherm Modeling for the Adsorption of Silver Ion from Aqueous Solution on a Superabsorbent Polymer, J. Mater. Environ. Sci., 2017, 8, 4705-4715.
- S. Agarry, O. Ogunleye, O. Ajani, Biosorptive removal of cadmium (II) ions from aqueous solution by chemically modified onion skin: batch equilibrium, kinetic and thermodynamic studies, Chemical Engineering Communications, 2015, 202, 655-673.
- C. Veloso, L. Filippov, I. Filippova, S. Ouvrard, A. Araujo, Technology, Adsorption of polymers onto iron oxides: Equilibrium isotherms, Journal of Materials Research, 2020, 9, 779-788.
- M. Uzqueda, A. Zornoza, J. R. Isasi, C. Martín, M. Sánchez, I. Vélaz, Interactions of terbinafine with β-cyclodextrin polymers: sorption and release studies, Journal of Inclusion Phenomena and Macrocyclic Chemistry, 2011, 69, 469-474.
DOI: http://dx.doi.org/10.13171/mjc02209271644amr
Refbacks
Copyright (c) 2022 Mediterranean Journal of Chemistry
