One pot synthesis of some novel indole derivatives and their antimicrobial activity
Abstract
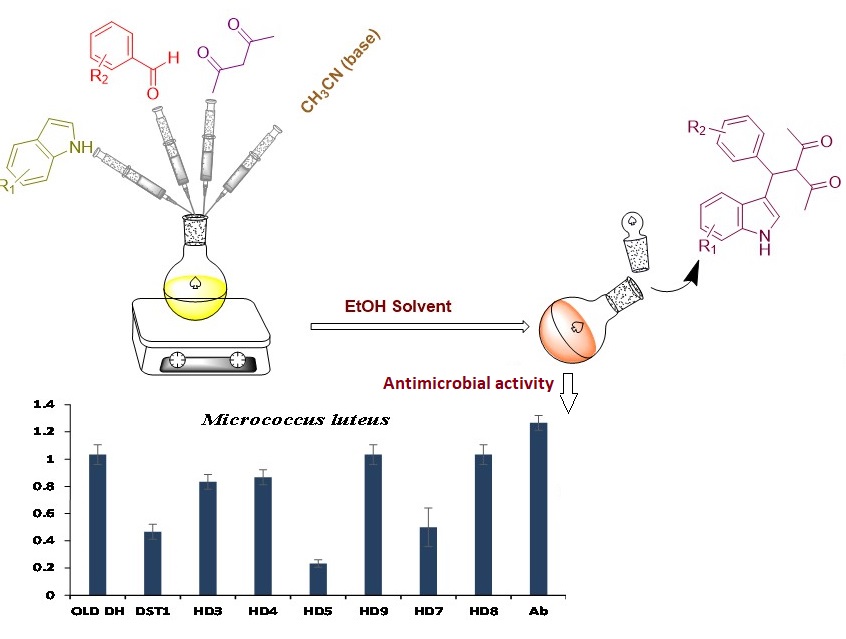
In this study, we describe the creation of a straightforward and incredibly effective protocol for the synthesis of new 3-Substituted indole derivatives. This multicomponent reaction involved indole, acetylacetone and active aldehyde derivatives in the presence of ethanol as solvent followed by acetonitrile as a base. We go through current developments in organic synthesis methodology for multicomponent reactions that produce substituted indole derivatives with a more environmentally friendly base catalytic condition, as well as the applications that go along with them. FT-IR, 1H-NMR and MS are used to characterise the synthesized compound of indole derivatives. Antibacterial activity of synthesized compounds has also been done.
Full Text:
PDFReferences
K. Hussain, J. Alam, A. Hussain, N. Kumar, A. Kumar, A. Raj, A Comprehensive Review on Indole Nucleus with Therapeutic Significance,
J. Pharm. Res. Ther., 2021, 1 (03), 149–160.
N.N. Makhova, L.I.; Belen’kii, G.A. Gazieva, I.L. Dalinger, S.L.; Konstantinova, V.V. Kuznetsov, A.N. Kravchenko, M.M. Krayushkin, O.A. Rakitin, A.M. Starosotnikov, L.L. Fershtat, S.A. Shevelev, V.Z. Shirinian, V.N. Yarovenko, Progress in the Chemistry of Nitrogen-, Oxygen- and Sulfur-Containing Heterocyclic Systems, Russ. Chem. Rev., 2020, 89 (1), 55–124.
M.M. Heravi, L. Mohammadkhani, Synthesis of Various N Heterocycles Using the Four-Component Ugi Reaction, 1st ed.; Elsevier Inc., 2020, 131.
D.I .Bugaenko, A.V. Karchava, A.M. Yurovskaya, Synthesis of Indoles: Recent Advances, Russ. Chem. Rev., 2019, 88 (2), 99–159.
F.B. Nikpassand, Potassium 2-Oxoimidazolidine-1,3-Diide: An Effective and New Catalyst for the Grinding Synthesis, Bull. Chem. Soc. Ethiop., 2018, 32 (2), 399–405.
T.L.S. Kishbaugh, Analisis Hubungan Modal Sosial Terhadap Keberdayaan Petani Karet, J. Online Mhs. Bid. Pertan., 2016, 03 (01),
–11.
J. Bariwal, L.G. Voskressensky, Recent Advances in Spirocyclization of Indole Derivatives, Chem. Soc. Rev., 2018, 47 (11), 3831–3848.
S. Itagaki, K. Kamata, K. Yamaguchi, N. Mizuno, Rhodium Acetate/Base-Catalyzed N-Silylation of Indole Derivatives with Hydrosilanes, Chem. Commun., 2012, 48 (74), 9269–9271.
H. Dangi, R. Das, S. Kashaw, Synthesis of Indoles Derivatives Using Metal Free Catalyst in Organic Reactions, Ankara Univ. Eczac. Fak. Derg., 2021, 45 (3), 615–630.
A. Roy, A.K. Bahe, A. Chanderiya, H. Dangi, P. Mishra, A.K. Mishra, R. Das, Synthesis of Nitrogen-and Oxygen-Containing Heterocyclic Compounds Using Nanocatalyst: A Review, J. Turkish Chem. Soc. Sect. A Chem., 2021, 8 (3), 851–862.
W.J. Yoo, M.G. Capdevila, X. Du, S. Kobayashi, Base-Mediated Carboxylation of Unprotected Indole Derivatives with Carbon Dioxide, Org. Lett., 2012, 14 (20), 5326–5329.
H.C. Zhang, H. Ye, A.F. Moretto, K.K. Brumfield, B.E. Maryanoff, Facile Solid-Phase Construction of Indole Derivatives Based on a Traceless, Activating Sulfonyl Linker, Org. Lett., 2000, 2 (1), 89–92.
A. Carpita, A. Ribecai, Microwave-Assisted Synthesis of Indole-Derivatives via Cycloisomerization of 2-Alkynylanilines in Water without Added Catalysts, Acids, or Bases, Tetrahedron Lett., 2009, 50 (49), 6877–6881.
H. Sachdeva, S. Sharma, Green Preparation and Structure Elucidation of Spiro Indole Derivatives Using Grindstone Technique, MOJ Bioorganic Org. Chem., 2017, 1 (5), 170–174.
X.B. Chen, S.L. Xiong, Z.X. Xie, Y.C. Wang, W. Liu, Three-Component One-Pot Synthesis of Highly Functionalized Bis-Indole Derivatives, ACS Omega, 2019, 4 (7), 11832–11837.
B. Jiang, T. Rajale, W. Wever, S. Tu, G. Li, Multicomponent Reactions for the Synthesis of Heterocycles, Chem. Asian J., 2010, 5, 2318–2335.
T. Masquelin, H. Bui, B. Brickley, G. Stephenson, J. Schwerkoske, C. Hulme, Sequential Ugi/Strecker Reactions via Microwave Assisted Organic Synthesis: Novel 3-Center-4-Component and 3-Center-5-Component Multicomponent Reactions, Tetrahedron Lett., 2006, 47 (17), 2989–2991.
D.G. Hall, T. Rybak, T. Verdelet, Multicomponent Hetero-[4 + 2] Cycloaddition/Allylboration Reaction: From Natural Product Synthesis to Drug Discovery, Acc. Chem. Res., 2016, 49 (11), 2489–2500.
S. Naureen, F. Chaudhry, N. Asif, M.A. Munawar, A. Khan, Four-Component, One-Pot Synthesis of Novel Conjugated Indole-Imidazole Derivatives, Iran. J. Chem. Chem. Eng., 2019, 38 (1), 57–64.
B. Banerjee, Recent Developments on Ultrasound-Assisted One-Pot Multicomponent Synthesis of Biologically Relevant Heterocycles, Ultrason. Sonochem., 2017, 35, 15–35.
Y. Zhu, J. Zhao, L. Luo, Y. Gao, H. Bao, P. Li, European Journal of Medicinal Chemistry Research Progress of Indole Compounds with Potential Antidiabetic Activity, Eur. J. Med. Chem., 2021, 223, 113665.
D.N. Turner, L. Edwards, A. Kornienko, L.V. Frolova, S. Rogelj, Synergistic Action of Substituted Indole Derivatives and Clinically Used Antibiotics against Drug-Resistant Bacteria, Future Microbiol., 2020, 15, 579–590.
W.M. Eldehna, F.M. Abo-Ashour, T. Al-Warhi, T.S. Al-Rashood, A. Alharbi, R.R. Ayyad, K. Al-Khayal, M. Abdulla, H.A. Abdel-Aziz,
R. Ahmad, R. El-Haggar, Development of 2-Oindolin-3-Ylidene-Indole-3-Carbohydrazide Derivatives as Novel Apoptotic and Anti-Proliferative Agents towards Colorectal Cancer Cells; Taylor & Francis, 2021, 36.
A.S. Salman, A.N. Mahmoud, A. Abdel-aziem, M.A. Mohamed, D.M. Elsisi, Synthesis, Reactions and Antimicrobial Activity of Some New 3-Substituted Indole Derivatives, Int. J. Org. Chem., 2015, 81–99.
P. Ashok, C.L. Lu, S. Chander, Y.T. Zheng, S. Murugesan, Design, Synthesis, and Biological Evaluation of 1-(Thiophen-2-Yl)-9H-Pyrido [3,4-b]Indole Derivatives as Anti-HIV-1 Agents, Chem. Biol. Drug Des., 2015, 85 (6), 722–728.
C. Wei, L. Zhao, Z. Sun, D. Hu, B. Song, Discovery of Novel Indole Derivatives Containing Dithioacetal as Potential Antiviral Agents for Plants, Pestic. Biochem. Physiol., 2020, 166, 104568.
D. Solanki, Nakra, A. Tiwari, A.K. Gupta, S. Gandhi, Synthesis, Characterization and Anti-Inflammatory Activity of Novel 1,5-Disubstituted Indole Derivatives, Eur. J. Mol. Clin. Med., 2020, 7 (11), 4622–4635.
B. Jasiewicz, W. Kozanecka-Okupnik, M. Przygodzki, B. Warżajtis, U. Rychlewska, T. Pospieszny, L. Mrówczyńska, Synthesis, Antioxidant and Cytoprotective Activity Evaluation of C-3 Substituted Indole Derivatives, Sci. Rep., 2021, 11 (1), 1–14.
P. Rajesab, P.W. Chavan, J.G. Badiger, P. Chanamshetty, Multicomponent One-Pot Synthesis of Novel Indole Analogues As Potent Antioxidant Agents, Asian J. Pharm. Clin. Res., 2022, 15 (6), 62–66.
C.K. Tudu, A. Bandyopadhyay, M. Kumar, D. Radha, S. Nandy, M. Ghorai, A.V. Gopalakrishnan, J. Proćków, A. Dey, Unravelling the Pharmacological Properties of Cryptolepine and Its Derivatives: A Mini-Review Insight, Naunyn. Schmiedebergs. Arch. Pharmacol., 2022, 2, 1–11.
G.A. Khan, J.A. War, A. Naikoo, G.A. Pandit, U.J. Das, R. Porous CuO Catalysed Green Synthesis of Some Novel 3-Alkylated Indoles as Potent Antitubercular Agents, J. Saudi Chem. Soc., 2018, 22 (1), 6–15.
T. Dhanya, G. Anjali Krishna, D.P. Savitha, A.A. Shanty, K.M. Divya, K. S. Priya, P.V.A. Mohanan, Review on the Synthesis and Biological Relevance of Benzo[b]Thiophene Derivatives, Phosphorus, Sulfur Silicon Relat. Elem., 2022, 1–17.
J. Vaca, F. Salazar, A. Ortiz, E. Sansinenea, Indole Alkaloid Derivatives as Building Blocks of Natural Products from Bacillus thuringiensis and Bacillus Velezensis and Their Antibacterial and Antifungal Activity Study, J. Antibiot., 2020, 5, 2–6.
P. Jain, D. Utreja, An Efficacious Synthesis of N-1–C‐3–Substituted Indole Derivatives and Their Antimicrobial Studies, J Heterocycl. Chem., 2019, 1, 1–8.
N. Chadha, O. Silakari, Indoles as therapeutics of interest in medicinal chemistry: Bird's eye view, AC SC. Eur. J. Med. Chem. 2017, 2, 4–90.
DOI: http://dx.doi.org/10.13171/mjc02302031664das-dangi
Refbacks
- There are currently no refbacks.
Copyright (c) 2023 Mediterranean Journal of Chemistry
