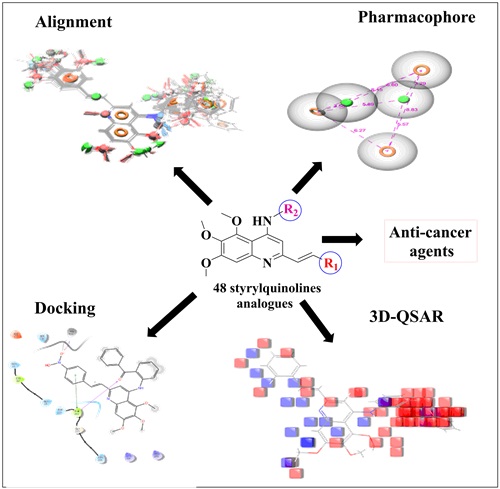
Structural insights into styrylquinolines analogs as tubulin polymerization inhibitors: In silico Approach
Abstract
Full Text:
PDFReferences
G.M. Cooper, The Cell: A Molecular Approach, 2nd edition. 2000.
N.G. Zaorsky, T.M. Churilla, B.L. Egleston, S.G. Fisher, J.A. Ridge, E.M. Horwitz, J.E. Meyer, Causes of death among cancer patients, Annals of Oncology, 2017, 28, 400-407.
A. Jemal, F. Bray, J. Ferlay, E. Ward, Global cancer statics, A cancer journal for clinicians, 2011, 61(2), 69-90.
H. Sung, J. Ferlay, R.L. Siegel, M.Laversanne, I. Soerjomataram, A. Jemal, F. Bray, Global Cancer Statistics 2020: GLOBOCAN Estimates of incidence and Mortality Worldwide for 36 Cancers in 185 Countries, A Cancer Journal for Clinicians, 2021, 71(3), 209-249.
M.E.A. de Kraker, A.J. Stewardson, S. Harbarth, Will 10 Million People Die a Year due to Antimicrobial Resistance by 2050?, Plos Medicine, 2016, 13(11), e1002184.
H. Khil, S.M. Kim, S.E. Hong, H.M. Gil, E. Cheon, D.H. Lee, Y.A. Kim, N. Keum, Time trends of colorectal cancer incidence and associated lifestyle factors in South Korea, Scientific Reports, 2021, 11, 2413.
S. Shrestha, R.S. Poudel, K.C. Bhuvan, B.K. Poudel, B. Sapkota, S. Sharma, A. Khadka, Price variation among different brands of anticancer medicines available in hospital pharmacies of Nepal, Journal of Pharmaceutical Policy and Practice, 2020.
I. Ojima, X. Wang, Y. Jing, C. Wang, Quest for Efficacious Next-Generation Taxoid Anticancer Agents and Their Tumor-Targeted Delivery, Journal of natural products, 2018, 81(3), 703-721.
A. Zarbo, A. Axelson, Common Cutaneous Side Effects of Anticancer Agents, Practical guide to dermatology, 2019, 289-306.
A.F. Hood, Cutaneous side effects of cancer chemotherapy, The Medical clinics of North America, 1986, 70(1), 187-209.
D. Sunil, P. Kamath, Indole-based tubulin polymerization inhibitors: An update on recent developments, Mini reviews in medicinal chemistry, 2016, 16(18), 1470-1499.
K.S. Chan, C.G. Koh, H.Y. Li, Mitosis-targeted anticancer therapies: where they stand, Cell Death & Disease, 2012.
J. Dinic, C. Rios-Luci, I. Karpaviciene, I. Cikotiene, M.P. Fernandes, J.M. Padron, CKTo353, a novel microtubule targeting agent, overcomes paclitaxel-induced resistance in cancer cells, Investigational New Drugs, 2020, 584-598.
L. Li, D. Quan, J. Chen, J. Ding, J. Zhao, L. Lv, J. Chen, Design, synthesis, and biological evaluation of 1- substituted-2-aryl imidazoles targeting tubulin polymerization as potential anticancer agents, European Journal of Medicinal Chemistry, 2019, 184, 111732.
A.L. Parker, M. Kavallaris, J.A. McCarroll, Microtubules and their role in cellular stress in cancer, Frontiers in Oncology, 2014, 4, 153.
M.A. Jordan, L. Wilson, Microtubules as a target for anticancer drugs, Nature Reviews Cancer, 2004, 4, 253-265.
L. Li, S. Jiang, X. Li, Y. Liu, J. Su, J. Chen, Recent advances in tri methoxyphenyl (TMP) based tubulin inhibitors targeting the colchicine binding site, European Journal of Medicinal chemistry, 2018, 151, 482-494.
W. Liu, G. Wang, Z. Peng, Y. Li, Design, Synthesis and Biological Evaluation of Novel 4-(4-Methoxynaphthalen-1-yl)-5-arylpyrimidin-2-amines as Tubulin Polymerization Inhibitors, Chemical and Pharmaceutical Bulletin, 2020, 68(12), 1184-1192.
Y. Wan, Y. Li, C. Yan, M. Yan, Z. Tang, Indole: A privileged scaffold for the design of anticancer agents, European Journal of Medicinal Chemistry, 2019, 183, 111691.
H. Guo, X. Li, Y. Guo, L. Zhen, An overview of tubulin modulators deposited in protein data bank, Medicinal Chemistry Research, 2019, 28, 927-937.
Y. Wang, H. Zhang, B. Gigant, Y. Yu, Y. Wu, X. Chen, Q. Lai, Z. Yang, Q. Chen, J. Yang, Structures of a diverse set of colchicine binding site inhibitors in complex with tubulin provide a rationale for drug discovery, The FEBS journal, 2015, 283(1), 102-111.
E. Mukhtar, V.M. Adhami, H. Mukhtar, Targeting Microtubules by Natural Agents for Cancer Therapy, Molecular Cancer Therapeutics, 2014.
A.F. Serpico, R. Visconti, D. Grieco, Exploiting immune-dependent effects of microtubule-targeting agents to improve efficacy and tolerability of cancer treatment, Cell Death & Disease, 2020.
K.E. Arnst, Y. Wang, D.J. Hwang, Y. Xue, T. Costello, D. Hamilton, Q. Chen, J. Yang, F. Park, J.T. Dalton, D.D. Mliier, W. Li, A Potent, Metabolically Stable Tubulin Inhibitor Targets the Colchicine Binding Site and Overcomes Taxane Resistance, Cancer Research, 2018.
V. Cermak, V. Dostal, M. Jelinek, L. Libusova, J. Kovar, D. Rosel, J. Brabek, Microtubule-targeting agents and their impact on cancer treatment, European Journal of Cell Biology, 2020, 99(4), m151075.
S. Marchal, A.E. Hor, M. Millard, V. Gillon, L. Bezdetnaya, Anticancer Drug Delivery: An Update on Clinically Applied Nanotherapeutics, Drugs, 2015, 75, 1601-1611.
S.B. Hastie, Interactions of colchicine with tubulin, Pharmacology & therapeutics, 1991, 51(3), 377-401.
Y. Lu, J. Chen, M. Xiao, W. Li, D.D. Miller, An Overview of Tubulin Inhibitors That Interact with the Colchicine Binding Site, Pharmaceutical Research, 2012, 29, 2943-2971.
E.C McLoughlin., N.M O’Boyle., “Colchicine-Binding Site Inhibitors from Chemistry to Clinic: A Review”, Pharmaceuticals, 2020, 13(1):8.
R.A. Stanton, K.M. Gernert, J.H. Nettles, R. Aneja, Drugs that target dynamic microtubules: A new molecular perspective, Medicinal Research Reviews, 2011, 31(3), 443-481.
X. Lv, C. He, C. Huang, G. Hua, Z. Wang, S.W. Remmenga, K.J. Rodabough, A.R. Karpf, J. Dong, J.S. Davis, C. Wang, G-1 Inhibits Breast Cancer Cell Growth via Targeting Colchicine-Binding Site of Tubulin to Interfere with Microtubule Assembly, Molecular cancer therapeutics, 2017, 16(6), 1080-1091.
P. Barbier, A. Dorleans, F. Devred, L. Sanz, D. Allegro, C. Alfonso, M. Knossow, V. Peyrot, J.M. Andreu, Stathmin and Interfacial Microtubule Inhibitors Recognize a Naturally Curved Conformation of Tubulin Dimers, Journal of Biological Chemistry, 2010, 285(41), 31672-31681.
S.Mirzaei, F.Eisvand, F.Hadizadeh, F.Mosaffa, A.Ghasemi, “Design, synthesis and biological evaluation of novel 5,6,7-trimethoxy-N-aryl-2-styrylquinolin-4-amines as potential anticancer agents and tubulin polymerization inhibitors”, Bioorganic Chemistry, 2020, 98:103711.
M. Afzal, J. Thathan, S. Jupudi, Pharmacophore modeling, atom-based 3D-QSAR, molecular docking and molecular dynamics studies on Escherichia coli ParE inhibitors, Computational Biology and Chemistry, 2020, 84, 107197.
V. Yele, M.A. Azam, S. Jupudi, Ligand-based pharmacophore modeling, in silico virtual screening, molecular docking, and molecular dynamic simulation study to identify novel Francisella tularensis ParE inhibitors, Chemical papers, 2020, 74, 4567-4580.
N.S. Tripuraneni, M.F. Azam, Pharmacophore modeling, 3D-QSAR and docking study of 2-phenyl pyrimidine analogs as selective PDE4B inhibitors, Journal of Theoretical Biology, 2016, 394, 117-126.
S. Bhansali, V.M. Kulkarni, Pharmacophore generation, 3D-QSAR, docking study, virtual screening studies of p38-α mitogen-activated protein kinase inhibitors: pyridopyridazine-6-ones (part 2), 2013, 2014, 4, 1-21.
http://gohom.win/ManualHom/Schrodinger_20152_docs/maestro/help_Maestro/phase/ato m_based_qsar.html
P. Kirubakaran, K. Muthusamy, K.H. Singh, S. Nagamani, Ligand-based pharmacophore modeling; atom-based 3D-QSAR analysis and molecular docking studies of phosphoinositide-dependent kinase-1 inhibitors, Indian J. Pharmaceut. Sci., 2012, 74, 141-51.
A. Golbraikh, A. Tropsha, Predictive QSAR modeling based on diversity sampling of experimental datasets for the training and test set selection, J. Comput. Aid. Mol. Des., 2002, 16, 357-369.
Ligprep, version 2.5, Schrödinger, LLC, New York, 2012.
Phase, version 4.4, Schrödinger, LLC, New York, 2012.
S.L. Dixon, A.M. Smondyrev, E.H. Knoll, S.N. Rao, D.E. Shaw, R.A. Friesner, PHASE: a new engine for pharmacophore perception, 3D QSAR model development, and 3D database screening: 1. Methodology and preliminary results, J. Comput. Aided Mol. Des., 2006, 20, 647-71.
S.J. Cottrell, V.J. Gillet, R. Taylor, D.J. Wilton, Generation of multiple pharmacophore hypotheses using multiobjective optimization techniques, Journal of computer-aided molecular design, 2004, 18(11), 665-682.
S. Nagamani, C. Kesavan, K. Muthusamy, E-Pharmacophore mapping and docking studies on Vitamin D receptor (VDR), Bioinformation, 2012, 8(5), 705-710.
Glide 6.7 User Manual, Schrodinger, LLC, May 2015.
https://www.schrodinger.com/science-articles/docking-and-scoring
X.Y. Meng, H.X. Zhang, M. Mezei, M. Cui, Molecular Docking: A powerful approach for structure-based drug discovery, Curr Comput Aided Drug Des., 2012, 7(2), 146-157.
S.D. Firke, A.M. Dhote, R.R. Patil, A.A. Shrikhedkar, S.J. Surana, Natural Antidiabetic Agents: Molecular Docking Study using the Extra Precision Method, Letters in Drug Design & Discovery, 2021, 18(2), 143-171.
K. Prabhu, M.M. Mahto, V.K. Gopalakrishnan, Virtual Screening, Molecular Docking and Molecular Dynamics Studies for Discovery of Novel Vegfr-2 Inhibitors, International Journal of Pharmaceutical and Clinical Research, 2014, 6(3), 221-229.
G.P. Mishra, D. Panigrahi, Molecular docking and ADMET study for searching multi-targeted antiviral compounds against SARS-CoV-2: A computational approach, International Journal of Applied Science and Engineering, 2020, 18(2), 004.
QikProp 3.5 User Manual, Schrodinger, LLC, September 2012.
S.M.Z. Hosen, M.S.H. Kabir, A. Hasanat, T.A. Chowdhury, N. Chakrabarty, S.K. Sarker, M.R. Habib, R. Dash, Docking and ADME/T analysis of silibinin as a potential inhibitor of EGFR kinase for ovarian cancer therapy, Journal of Applied Pharmaceutical Science, 2016, 6(08), 001-005.
DOI: http://dx.doi.org/10.13171/mjc02304201689kashaw
Refbacks
- There are currently no refbacks.
Copyright (c) 2023 Mediterranean Journal of Chemistry
