Crystal Structure and DFT Computations of a Solid-State Solution of Mixed Mononuclear Cu(II)/Co(II) Complex
Abstract
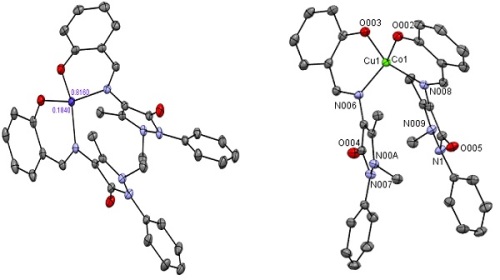
A novel mixed molecular Cu (II) and Co (II) complexes [Cu L2]0.82 and [CoL2]0.18 (1) where HL = (4-salicylaldaimine antipyrine) derived from 4-aminoantipyrine and 2-hydroxybenzaldehyde, was isolated as a green solid and characterized by Fourier Transform Infrared Spectroscopy (FTIR), Ultra-Violet Visible spectroscopy (UV-Vis) and Single-crystal x-ray diffraction. Compound 1 is a solid-state solution that contains a statistical mixture of two different molecular complexes: 82% of a 17-electron Cu (II) complex and 18% of a 15-electron Co (II) complex. By looking at the structure, it seems that the two metal ions occupy the same metal site, which is not true; at least a small distance between them exists, verified using the Density functional theory (DFT). A divalent metal cation binds four coordination sites in each molecular complex from two corresponding bidentate Schiff base ligands. Each metal atom is coordinated with two nitrogen and two oxygen atoms of the Schiff base ligand in a geometry much closer to pseudo-tetrahedral than planar. DFT calculations were used to validate the trends observed in bond lengths and angles in this structure. By using the DFT methods, it is seen that there are no significant differences in the experimental values obtained by single-crystal X-ray diffraction. It should be noted that the experimental results belong to the solid phase, while the theoretical calculations belong to the gas phase.
Full Text:
PDFReferences
- S. Alina, B. Alexandra, Advances and Biomedical Applications of Schiff-Base Ligands and Their Metal Complexes, Crystals, 2022, 12(10), 1436-1446.
- S.S. Mukhtar, A.S. Hassan, A.S., Morsy, N.M., Hafez, T. S. Hassaneen, H. M., Saleh, Overview on synthesis, reaction, applications and biological activities of Schiff bases, Egypt, J. Chem., 2021, 64, 6541-6554.
- B. D. Nath, M. Islam, M. Karim, R. Rahman, A.A. Shaikh, P.E. Georghiou, M. Menelaou, Recent progress in metal-incorporated a cyclic Schiff-base derivative. Biological Aspects, Chem. Sel., 2022, 7, e20210429.
- K. Monal, S. Mistris, Schiff base based metal complexes: A review of the catalytic activity on Aldol and Henry reaction. Comm. Inorg. Chem. 2022, 77-105.
- S.S. Maria, C.D. Jessica, L. Dominica, C. Alessia, M. Annalusia, R. Camillo, S. Carmela, H. El-kashef and I. Pasqula, Recent studies on mononuclear and binuclear metal complexes with Schiff bases, focusing primary on antimicrobial activity, Int. J. Mol. SCI., 2022, 23(23), 14840.
- D. Chiaramonte, J.M. Steiner, J.D. Broussard, K. Baer, S.Gumminge, E.M. Moeller, D.A. Williams, R. Shumway, Use of a 13C- aminopyrine blood test: First clinical impressions, Can J Vet Res., 2003, 67, 183-188.
- J. Juvansinh J. B Mitesh, D.D. Gondaliya, M.M Shah, Synthesis, spectral characterization and biological activity studies of Schiff’s base of 1,5-dimethyl-2-phenyl-2,3-dihydro-1H-pyrazol-4-amine and its metal complexes., -world scientific news, 47(2), 2016, 123-150.
- B. Anupama, M. Padmaja, C.G. Kumari, Synthesis, characterization, biological activity and DNA binding studies of metal complexes with 4-aminoantipyrine Schiff base ligand, Eur. J. Chem., 2012, 9(1), 389-400.
- G.S. Kurdekar, M. Sathisha, S. Budagumpi, N.V. Kulkarni, V.K. Revankar, D. Suresh, 4-Aminoantipyrine-based Schiff-base transition metal complexes as potent anticonvulsant agents, Med Chem. Res., 2012, 21, 2273-2279.
- A.P. Mishra, R. Mishra, R. Jain, S. Gupta, Synthesis of new VO (II), Co (II), Ni (II) and Cu (II) complexes with Isatin-3-chloro-4-floroaniline and 2-pyridinecarboxylidene-4-aminoantipyrine and their antimicrobial studies, Mycobiology, 2012, 40, 20-26.
- N. Raman, S.J. Raja, A. Sakthivel, Transition metal complexes with Schiff-base ligands: 4-aminoantipyrine based derivatives–a review, J. Coord. Chem., 2009, 62, 691-709.
- M.E. Hossain, M.N. Alam, J. Begum, A.M Akbar, M. Nazimuddin, F. E.Smith, R.C. Hynes, Copper (II) Complexes of the 2- Benzoylpyridine Schiff bases of S-methyl- and S-benzyldithiocarbazate, Inorg Chim Acta., 1996, 249, 207-213.
- E.M. Hodnett, W.J. Dunn, Structure-antitumor activity correlation of some Schiff bases, J. Med. Chem., 1970, 13, 768–770.
- H. Kargar, F. Aghaei-Meybodi, R. Behjatmanesh-Ardakani, M. R Elahifardc V.Torabi, M.Fallah-Mehrjardi, M.Nawaz Tahir M.Ashfaq K.S Munawar, Synthesis, crystal structure, theoretical calculation, spectroscopic and antibacterial activity studies of copper (II) complexes bearing bidentate Schiff base ligands derived from 4-aminoantipyrine: Influence of substitutions on antibacterial activity, Journal of Molecular Structure, 2021, 1230, 129908.
- R.M. El-mehdawi, A.N. Eldewik, K.M. Kreddan, S.H. El-hamruni, H. Ben-Hussien, A.A. HitchcShabash, Synthesis and crystal structure of Bis- [Co (L)(NCS)(MeOH)]where (LH=4. (Salicylaldiminato) antipyrine, Jordan J. Chem., 2010, 5, 157.
- R.M. El-mehdawi, A.N. El-dewik, M.M. Ben-Yunes, F.A. Treish, R.G. Abuhmaiera, D.R.
Poleti, J. Synthesis Characterization and Crystal Structure of [Co4(CH3CO2)2L4]2[BPh4]4.0.5H2O, where HL= 4-(Salicyaldiminato) antipyrine, J Crystallography, 2014, 481572, 1-6.
- R.M. Elmehdawi, M.N. El-kaheli, G.R. Abuhmaiera, F.A. Treish, C. Bazzicalupi, A. Caneschi, A. Amjad, Synthesis, M.M. Ben-Younes Crystal Structure, and Magnetic Properties of a New Mixed Metal (Co(II), Ni(II)) Cubane, Materials, 2017, 10, 178.
- T. Ziegler, Density functional theory as a practical tool for the study of elementary reaction steps in organometallic chemistry, Pure and Applied Chemistry, 1991, 63, 873–878.
- P.M. Gill, B.G. Johnson, J. A. Pople, M. J. Frisch, The performance of the Becke-Lee-Yang-Parr (B-LYP) density functional theory with various basis sets, Chemical Physics Letters, 1992, 197, 4-5, 499–505.
- Y.M. Chumakov, B.Y. Antosyak, M.D. Mazus, V. I. Taspkov, M.N.Samus, Crystal structure of N-(salicylidene)-4-amino-2,3-dimethyl-1-phenyl-3-pyrazolin-5-one, Struct. Chem., 2000, 41, 905–909.
- G.M.Sheldrick, Crystal structure refinement with SHELXL, Acta Crystallogr. C Struct. Chem., 2015, 71, 3–8.
- T.R. Schneider, G.M.Sheldrick, Substructure solution with SHELXD, Acta Crystallogr. D Biol. Crystallogr., 2002, 58, 1997-1772.
- J.L. Farrugia, ORTEP-3 for Windows—A version of ORTEP-III with a Graphical User. Interface (GUI), J. Appl. Crystallogr., 1997, 30, 565.
- M.A. Nardelli, System of Fortran routines for calculating molecular structure parameters from the results of crystallography. J. Appl. Crystallogr., 1995, 28, 659.
- T.Roso, S. Pasculescu, V. Lazar, C. Chifiruc, R. Cernat, Copper(II) complexes with ligand derivatives. Crystal Structure of N-(salicylidene)-4-amino-2,3-dimethyl-1-phenyl-3-pyrazolin-5-one. J. Struct. Chem., 2011, 41, 905-909.
- R.A. Gaussian, M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone,
B. Mennucci, G.A. Petersson, Gaussian, Inc, Wallingford CT, 2009.
- S. El-t Ashoor, H.B. Shawish, Synthesis, X-Ray Crystallography and DFT Studies of Ni(II) Complex with Tetradentate, Physics and Materials Chemistry, 2015, 3 (1), 7-11.
- S. El-t Ashoor, R.A. Abokhater, L. Belkhiri, S.A. Gadir, Spectroscopy and DFT studies on Cr(III) complexes with saccharides, 8 hydroxyquinoline and their biological activity, Journal of Molecular Structure, 2023, 1281, 135134.
DOI: http://dx.doi.org/10.13171/mjc02305291700elmehdawi-ashoor
Refbacks
- There are currently no refbacks.
Copyright (c) 2023 Mediterranean Journal of Chemistry
