Phytochemical analysis, evaluation of the antioxidant and antiplasmodial activities of the ethanolic extract of Ficus elastica Roxb. ex Hornem. (Moraceae) lianas
Abstract
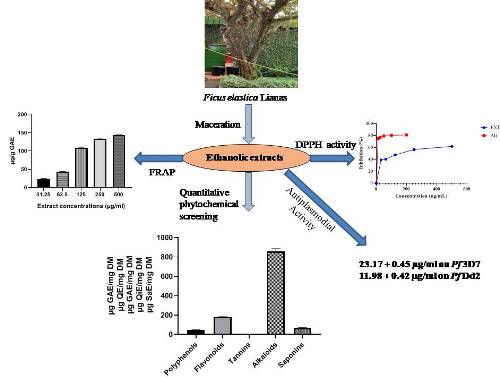
Ficus elastica Roxb. ex Hornem, also called the Rubber fig tree, is a plant of the Moraceae family used in traditional medicine to treat allergies and skin infections. Our study aims to perform the quantitative screening and the evaluation of the antioxidant and anti-plasmodial activities of the ethanolic extract of Ficus elastica lianas. The estimation of total polyphenols, flavonoids, tannins, alkaloids, and saponins has been evaluated by different methods described in the literature. The evaluation of the antioxidant activity was carried out using the DPPH scavenging method and that of the ferric-reducing antioxidant power (FRAP). The antiplasmodial activity was tested on the Pf 3D7 strain (sensitive to Chloroquine and artemisinin), and Pf Dd2 (chloroquine-resistant and sensitive to artemisinin), and the inhibitory concentration 50 (IC50) was determined. Phytochemical screening revealed the presence of alkaloids, flavonoids, saponins, polyphenols, and tannins with respective contents of 855.2 µg QiE/mg D.M., 179.99 µg Q.E./mg D.M., 68.24 µg SaE/mg D.M., 46.46 µg GAE/mg D.M. and 0.11 µg TAE/mg D.M. The extract of Ficus elastica lianas showed antioxidant activity with an IC50 = 25.30 µg/ml compared to that of gallic acid, which was 5.06 µg/ml. At 31,25 µg/ml, a 22,49 µg/g GAE concentration could reduce Fe3+ to Fe2+. It also showed good antiplasmodial activity (IC50: 11.98 ± 0.42 µg/ml) on strain Dd2 and moderate activity (IC50: 23.17 ± 0.45 µg/ml) on strain 3D7. On the Pf Dd2 strain, the extract was more active than Chloroquine and artemisinin, while on the Pf 3D7 strain, the extract showed better activity than Chloroquine but less than artemisinin. These results demonstrate that the ethanolic extract of Ficus elastica lianas has antioxidant and antiplasmodial properties, probably due to its high alkaloids and flavonoids. It could be a natural alternative for discovering new antioxidant and antimalarial drugs.
Full Text:
PDFReferences
J. Haleng, J. Pincemail, J.O. Defraigne, C. Charlier, J.P. Chapelle, Oxidative Stress, Rev. Med. Liege, 2007, 62, 628-638.
M. Suhaj, Spice antioxidants isolation and their anti-radical activity: a review, J. Food Compost. Anal., 2006, 19, 531-537. https://doi.org/10.1016/j.jfca.2004.11.005.
M.B. Tadhani, V.H. Patel, R. Subhash, In vitro antioxidant activities of Stevia rebaudiana leaves and callus, J. Food Compost. Anal., 2007, 20,
–329. https://doi.org/10.1016/j.jfca.2006.08.004.
C. Koechlin-Ramonatxo, Oxygen, oxidative stress and antioxidant supplementation, or another way for nutrition in respiratory diseases, Nutr. Clin. Metab., 2006, 20, 165-177. https://doi.org/10.1016/j.nupar.2006.10.178.
D.L. Vârban, M. Duda, R. Vârban, S. Muntean, Research concerning the Organic Technology for Satureja hortensis L, Cultue, Bull. Univ. Agric. Sci. Vet. Med. Agri., 2009, 66, 1843-5386. https://doi.org/10.15835/buasvmcn-agr:4236.
World Health Organization, Global Malaria Programme/World Malaria Report 2022, 2021, [Accessed on the 5th November 2023]. Available : http://who.int/fr/teams/global-malaria-programme/reports/world-malaria-report-2022.
Malaria, Nearly every minute, a child under 5 dies of Malaria, 2023, [Accessed on the 5th November 2023]. Available : http://data.unicef.org/topic/child-health/malaria/.
P. Nyam, The fight against Malaria in Cameroon: autopsy of a plural phenomenon in a context of hyper endemicity, 2020, [Accessed on the 5th November 2023]. Available : http:// hal.science/hal-03078921.
D.P.B. Lakouéténé, G. Ndolngar, B. Berké, J.M. Moyen, E.K. Komba, I. Zinga, S. Silla, J. Millogo-Rasolodimby, P. Vincendeau, J.-L. Syssa, Magalé, O.G. Nacoulma-Ouedraogo, R. Laganier, A. Badoc, C. Chèze, Ethnobotanical survey of plants used in the treatment of Malaria in Bangui, Bull. Soc. Pharm. Bord., 2009, 148, 123-138.
J.E.T. Mbosso, G.T. Wéa, C.N. Ngo, Y. Fouekeng, E.M. Mpondo, J.C.N. Assob, Anti-proliferative, antimicrobial, antiplasmodial, and oral acute toxicity of Ficus elastica Roxb. Ex Hornem lianas, Invest. Med. Chem. Pharmacol., 2023, 6, 77. https://doi.org/10.31183/imcp.2023.00077.
Y. Preeti, J. Abhishek, K. Gaurav, K. Loganathan, R. Bhaskar, Phytochemical composition and antioxidant activity of Ficus elastica Roxb. (Moraceae) leaves, Res. J. Pharm. Technol., 2015, 8, 259-264. https://doi.org/10.5958/0974-360X.2015.00043.8.
J.E.T. Mbosso, J.C.N. Assob, F. Meyer, N. Lenta, S. Ngouela, B. Lallemand, V. Mathieu, P. Van Antwerpen P, A.L. Njunda, D. Adiogo,
E. Tsamo, Y. Looze, R. Kiss, R. Wintjens, Ceramide, cerebroside and triterpenoid saponin from the bark of aerial roots of Ficus elastica (Moraceae), Phytochemistry, 2012, 8, 95-103. https://doi.org/10.1016/j.phytochem.2012.07.010.
J.E.T. Mbosso, X.N. Siwe, E.L. Nguemfo, F. Meyer, A.D. Djoukoué, P. Van Antwerpen, S. Ngouela, E. Tsamo, A.E.M. Mpondo, J.C. Vardamides, G.A.B. Azebaze, R. Wintjens, Identification of compounds with anti-proliferative activity from the wood of Ficus elastica Roxb. Ex Hornem. aerial roots, Fitoterapia, 2016, 112, 65–67. https://doi.org/10.1016/j.fitote.2016.05.002.
A.A. Adesokan, M.T. Yakubu, B.V. Owoyele, M.A. Akanji, A.O. Soladoye, O.K. Lawal, Effect of administration of aqueous and ethanolic extracts of Enantia chlorantha stem bark on brewer’s yeast-induced pyresis in rats, Afr. J. Biochem. Res., 2008, 2(7), 165-169.
V.L. Singleton, J.J.A. Rossi, Colorimetry of total phenolics with phosphomolybidic-phosphotungstic acid reagents, Am. J. Enology Viticult., 1965, 16, 144-158.
O.A. Aiyegoro, A.I. Okoh, Preliminary phytochemical screening and In vitro antioxidant activities of the aqueous extract of Helichrysum longifolium D.C. BMC Complement, Altern. Med., 2010, 10(1), 1-8. https://doi.org/10.1186/1472-6882-10-21.
B.S. Sun, J.M. Ricardo-da-Silva, M.I. Spranger, Critical factors of vanillin assay for catechins and proanthocyanidins, J. Agri. Food Chem., 1998, 46(10), 4267-4274. https://doi.org/10.1021/JF980366J.
E.H.G.Diouf, A. Samb, O. Sylla, A.E. Kafia, M. Diop, D. Seck, K. Nguessan, Phytochemical and insecticidal test of three organic extracts of ficus thonningii leaves on Callosobruchus maculatus Fabricius, Int. J. Biol. Chem. Sci., 2014, 8(6), 2588-2596. https://doi.org/10.4314/ijbcs.v8i6.20.
S. Hiai, H. Oura, T. Nakajima, Color reaction of some sapogenins and saponins with vanillin sulfuric acid, Planta Med., 1976, 29, 116-122. https://doi.org/10.1055/s-0028-1097639.
P. Kefalas, S. Kallithraka, I. Parejo, A comparative study on the in vitro anti-radical activity and hydroxyl free radical scavenging activity in aged red wines, Food Sci. Technol. Int., 2003, 9,
-387. https://doi.org/10.1177/1082013203040080.
P. Vrabl, C.W. Schinagl, D.J. Artmann, Fungal growth in batch culture–what we could benefit if we start looking closer, Front Microbiol., 2019, 10, 2391-2396. https://doi.org/10.3389/fmicb.2019.02391.
W. Trager W, J.B. Jensen, Human malaria parasites in continuous culture, Science, 1976, 193, 673-675. https://doi.org/10.1126/science.781840.
M.G. Vossen, S. Pferschy, P. Chiba, H. Noedl, The SYBR Green I malaria drug sensitivity assay: performance in low parasitemia samples, Am. J. Trop. Med. Hyg., 2010, 82, 398-401. https://doi.org/10.4269/ajtmh.2010.09-0417.
C.N. Ginting, I.N.E. Lister, E. Girsang, D. Riastawati, H.S.W. Kusuma, W. Widowati, Antioxidant activities of Ficus elastica leaves ethanol extract and its compounds, Mol. Cell. Biomed. Sci., 2020, 4, 27-33. https://doi.org/10.21705/mcbs.v4i1.86.
S.S. El-Hawary, G.M. Wassel, B.S. El-Menshawi, N.A. Ibrahim, K. Mahmoud, M.M. Ayoub, Antitumor and antioxidant activity of Ficus elastica Roxb. and Ficus bengalensis Linn. Family Moraceae, World Appl. Sci. J., 2012, 19, 1532-1539. https://doi.org/10.5829/idosi.wasj.2012.19.11.2796.
C. Samaniego-Sánchez, A. González, M. Garcìa-Parrilla, J. Granados, H. Garcia de la serrana, M. Martinez, Different radical scavenging tests in virgin olive oil and their relation to the total phenol content, Anal. Chim. Acta., 2007, 593, 103-107. https://doi.org/10.1016/j.aca.2007.04.037.
F.N. Njayou, P.T. Moundipa, A.N. Tchana, B.T. Ngadjui, F.M. Tchouanguep, Inhibition of merosomal lipid peroxidation and protein oxydation by extracts from plants used in Bamoun folk medecine (Cameroon) against hepatitis, Afr. J. Tradit. Complement. Altern. Med., 2008, 5, 278-289. https://doi.org/10.4314/ajtcam.v5i3.31284.
N. Singh, N.K. Kaushik, D. Mohanakrishnan, S.K. Tiwari, D. Sahal, Antiplasmodial activity of medicinal plants from Chhotanagpur plateau, Jharkhand, India, J. Ethnopharmacol., 2015, 165, 152-162. https://doi.org/10.1016/j.jep.2015.02.038.
B.S. Kumulungui, Analysis of the pharmacological and genetic bases of resistance to Malaria. [PhD Thesis]. Ouagadougou: University of Ouagadougou, University of Aix-Marseille II; 1998. [Accessed on the 5th November 2023], Available:
https://www.beep.ird.fr/collect/uouaga/index/assoc/M08378.dir/M08378.pdf.
J. Bero, Evaluation of the antiparasitic activity of plants used in traditional medicine in Benin and identification of active ingredients, Pharmacy Thesis, Louvain: Catholic University of Louvain, 2012. Available: http://hdl.handle.net/2078.1/111492.
A.M. Lehane, K.J. Saliba, Common dietary flavonoids inhibit the growth of the intraerythrocytic malaria parasite, BMC Res. Notes, 2008, 1, 26. https://doi.org/10.1186/1756-0500-1-26.
E. Marliana, R. Hairani, T.S. Tjahjandarie, M. Tanjung, Antiplasmodial activity of flavonoids from Macaranga tanarius leaves, IOP Conf. Series: Earth Environ. Sci., 2018, 144, 012011. https://doi.org/10.1088/1755-1315/144/1/012011.
DOI: http://dx.doi.org/10.13171/mjc02401291768mbosso_teinkela
Refbacks
- There are currently no refbacks.
Copyright (c) 2024 Mediterranean Journal of Chemistry
