Aluminum and Mild Steel Inhibition Investigation Using Trithiocyanuric Acid: Insights from Density Functional Theory (DFT)
Abstract
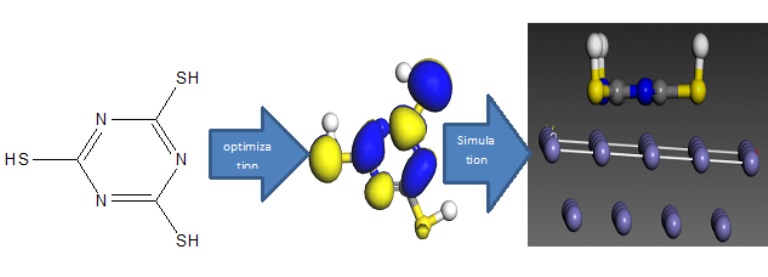
The study investigates the corrosion inhibition properties of Trithiocyanuric Acid (TTCA) on Aluminum and mild steel using Density Functional Theory (DFT) and Molecular Dynamics (MD) simulations. The electronic properties of TTCA, including the highest occupied molecular orbital (EHOMO) of -7.617 eV and the lowest unoccupied molecular orbital (ELUMO) of -4.301 eV, yield an energy gap of 3.316 eV. The calculated absolute electronegativity was 5.959 eV, global hardness obtained was 1.658 eV, global softness was 0.603136 eV, global electrophilicity index was 1.449891 eV, and nucleophilicity was 0.689707 eV. The energy of back donation was also 0.689707 eV. The charge transfer parameters, ΔNFe and ΔNAl, are also 0.862989 eV and -0.21653 eV, respectively, indicating effective interaction with both metal surfaces. Functional groups in TTCA contribute significantly to its corrosion inhibition performance by facilitating alignment on the metal surfaces. The interaction mechanism is characterized as physisorption, with binding energy values falling within the range typical for this adsorption type. The results suggest that TTCA is a suitable corrosion inhibitor for Aluminum and mild steel, making it a promising molecule for industrial applications where metal protection is essential.
Full Text:
PDFReferences
E. Kroke, C. Posern, C.C. Höhne, U. Böhme, C. Vogt, Synthesis of Thiocyameluric Acid C6N7S3H3, its Reaction to Alkali Metal Thio¬cyamelurates and Organic Tris(dithio)cyamelurates, Chemistry-A European Journal, 2019, 25, 15555-15564.
S. Hong, W. Chen, Y. Zhang, H.Q. Luo, M. Li, N.B. Li, Investigation of the inhibition effect of trithiocyanuric acid on corrosion of copper in 3.0wt.% NaCl, Corrosion Science, 2013, 66,
–314.
W. Chen, S. Hong, B. Xiang, H.Q. Luo, M. Li, N.B. Li, Corrosion inhibition of copper in hydrochloric acid by coverage with trithiocyanuric acid self-assembled monolayers, Corrosion Engineering, Science and Technology, 2013, 48, 98–107.
N.B. Iroha, C.U. Dueke-Eze, T.M. Fasina, V.C. Anadebe, L. Guo, Anticorrosion activity of two new pyridine derivatives in protecting X70 pipeline steel in oil well acidizing fluid: experimental and quantum chemical studies, J. Iran. Chem. Soc., 2021, 19, 1–16.
R. Songbo, G. Ying, K. Chao, G. Song, X. Shanhua, Y. Liqiong, Effects of the corrosion pitting parameters on the mechanical properties of corroded steel, Constr. Build. Mater., 2021, 272, 121941.
B. El Ibrahim, J.V. Nardeli, L. Guo, An overview of corrosion, ACS Symp Ser., 2021, 1403, 1–19.
M. Conradi, Development of mechanical, corrosion resistance, and antibacterial properties of steels, Materials, 2021, 14, 1–2.
K.P. Dahal, J.N. Timilsena, M. Gautam, J. Bhattarai, Investigation on probabilistic model for corrosion failure level of buried pipelines in Kirtipur urban areas (Nepal), J. Fail. Anal. Prev., 2021, 21, 914–926.
M.M. Hanoon, A.M. Resen, A.A. Al-Amiery, A.A.H. Kadhum, M.S. Takriff, Theoretical and experimental studies on the corrosion inhibition potentials of 2-((6-methyl-2-ketoquinolin-3-yl) methylene) hydrazinecarbothioamide for mild steel in 1 M HCl, Prog. Color. Color. Coat., 2021, 15, 21–33.
N.O. Obi-Egbedi, N.D. Ojo, Computational studies of the corrosion inhibition potentials of some derivatives of 1H-imidazo[4,5-F] [1,10] phenanthroline, J. Sci. Res., 2015, 14, 50–56.
F. Iorhuna, M.A. Ayuba, A.T. Nyijime, M. Sani, H. Abdulmumini, J.O. Oyeyode, A Comparative Computational Stimulation Studies on Corrosion Inhibition and Adsorptive Qualities of Coumarin Derivative on Iron, Zinc, Copper and Aluminium, Eurasian J. Sci. Tech., 2024, 4(1), 44-56.
S. Asadi, G.M. Ziarani, The molecular diversity scope of 1,3-indandione in organic synthesis, Mol Divers, 2016, 20(1), 111–152.
F. Iorhuna, M.A. Ayuba, A.T. Nyijime, H. Muhammadjamiu, M. Ibrahim, Comparative Study of Halogen Substituted Isocyanatophosphine as an Adsorptive Inhibitor on Al (110) Crystal Surface, Using Density Functional Theory, Prog. Chem. Biochem. Res, 2023, 6(3), 211-228.
I.B. Obot, D.D. Macdonald, Z.M. Gasem, Density functional theory (DFT) as a powerful tool for designing new organic corrosion inhibitors, Corros. Sci., 2015, 99, 1–30.
A. Dehghani, G. Bahlakeh, B. Ramezanzadeh, A.H. Mostafatabar, Construction of a zinc-centered metal–organic film with high anti-corrosion potency through covalent-bonding between the natural flavonoid-based molecules (Quercetin)/divalent-zinc: Computer modeling (integrated-DFT&MC/MD)/electrochemical-surface assessments, J. Ind. Eng. Chem., 2020, 88, 382–395.
N.S. Abdelshafi, M.A. Sadik, A.M. Shoeib, S.A. Halim, Corrosion inhibition of Aluminum in 1 M HCl by novel pyrimidine derivatives, EFM measurements, DFT calculations and MD simulation, Arab. J. Chem., 2022, 15(1), 103459.
A. Abdullahi Muhammad, N. Aondofa Thomas, N. Usman Shehu, F. Iorhuna, Exploring the Inhibition Potential of Carbamodithionic acid on Fe (111) Surface: A Theoretical Study, J. Eng. Ind. Res., 2024, 4(4), 201-210.
B. El Ibrahimi, A. Jmiai, K. El Mouaden, R. Oukhrib, A. Soumoue, S. El Issami, L. Bazzi, Theoretical evaluation of some α-amino acids for corrosion inhibition of copper in acidic medium: DFT calculations, Monte Carlo simulations and QSPR studies, J. King Saud Univ. Sci., 2020, 32, 163–171.
M.E. Mashuga, L.O. Olasunkanmi, H. Lgaz, E.S.M. Sherif, E.E. Ebenso, Aminomethylpyridazine isomers as corrosion inhibitors for mild steel in 1 M HCl: Electrochemical, DFT and Monte Carlo simulation studies, J. Mol. Liq., 2021, 344, 117882.
R. Hsissou, F. Benhiba, O. Dagdag, M. El Bouchti, K. Nouneh, M. Assouag, A. Elharfi, Development and potential performance of prepolymer in corrosion inhibition for carbon steel in 1.0 M HCl: outlooks from experimental and computational investigations, J. Colloid Interface Sci., 2020, 574, 43–60.
V.S. Sastri, M. Elboujdaini, J.R. Perumareddi, Utility of quantum chemical parameters in the rationalization of corrosion inhibition efficiency of some organic inhibitors, Corrosion, 2005, 61, 933–942.
T. A. Nyijime , H. F. Chahul, P. I. Kutshak, A. M. Ayuba, F. Iorhuna, V. Okai, and A. Hudu. Theoretical Investigation of Aluminum Corrosion Inhibition Using Chalcone Derivatives. Mediterranean Journal of Chemistry 2024, 14(1), 58-68. DOI: http://dx.doi.org/10.13171/mjc02402281774nyijime.
N. Hossain, M.A. Chowdhury, M. Kchaou, An overview of green corrosion inhibitors for sustainable and environment friendly industrial development, J. Adhes. Sci. Technol., 2021, 35, 673–690.
Y. Qiang, L. Guo, H. Li, X. Lan, Fabrication of environmentally friendly Losartan potassium film for corrosion inhibition of mild steel in HCl medium, Chem. Eng. J., 2021, 406, 126863.
M. Palomar-Pardavé, M. Romero-Romo, H. Herrera-Hernández, M.A. Abreu-Quijano, N.V. Likhanova, J. Uruchurtu, J.M. Juárez-García, Influence of the alkyl chain length of 2- amino 5 alkyl 1,3,4 thiadiazole compounds on the corrosion inhibition of steel immersed in sulfuric acid solutions, Corros. Sci., 2012, 54, 231–243.
S. Hadisaputra, A.A. Purwoko, S. Hamdiani, Substituents effects on the corrosion inhibition performance of pyrazolone against carbon steels: quantum chemical and Monte Carlo simulation studies, Int. J. Corros. Scale Inhib., 2021, 10, 419–440.
J.D. Pietrzyk, C.W. Frank, Analytical Chemistry, Academic Press, 1979, 410-424. https://doi.org/10.1016/B978-0-12-555160-1.50022-8.
J.S. Amoko, O.F. Akinyele, O.E. Oyeneyin, D.S. Olayanju, Corrosion inhibitive potentials of (E)-5-((4-Benzoylphenyl) Diazenyl)-2-hydroxybenzoic acid on mild steel surface in 0.5 M HCl-experimental and DFT calculations, J Turkish Chem Soc Sect A Chem., 2021, 8(1), 343–362.
J.I. Martinez-Araya, Why is the dual descriptor a more accurate local reactivity descriptor than Fukui functions?, J. Math. Chem., 2015, 53(2), 451–465.
E.O. Oluwatoba, N.D. Ojo, N. Ipinloju, E.B. Agbaffa, A.V. Emmanuel, Investigation of the corrosion inhibition potentials of some 2‑(4‑(substituted) arylidene)‑1H‑indene‑1,3‑dione derivatives: density functional theory and molecular dynamics simulation, Beni-Suef. Univ. J. Basic. Appl. Sci., 2022, 11, 132
M.B. Oulhaoua, M. El Hafi, S. Zehra, L. Eddaif, A.A. Alrashdi, S. Lahmidi, L. Guo, J.T. Mague, H. Lgaz, Colloids and Surfaces A : Physicochemical and Engineering Aspects Synthesis, structural analysis and corrosion inhibition application of a new indazole derivative on mild steel surface in acidic media complemented with DFT and MD studies, Colloids Surf A Physicochem Eng Asp., 2021, 617, 126373.
H. Lgaz, S. Masroor, M. Chafiq, M. Damej, A. Brahmia, R. Salghi, M. Benmessaoud, I.H. Ali, M.M. Alghamdi, A. Chaouiki, I.M. Chung, Evaluation of 2-mercaptobenzimidazole derivatives as corrosion inhibitors for mild steel in hydrochloric acid, Metals, 2020, 10(3),1–14.
A. Zarrouk, B. Hammouti, T. Lakhlifi, M. Traisnel, H. Vezin, F. Bentiss, New 1H-pyrrole-2,5-dione derivatives as efficient organic inhibitors of carbon steel corrosion in hydrochloric acid medium: electrochemical, XPS and DFT studies, Corros. Sci., 2015, 90, 572–584.
O. Oyeneyin, D. Akerele, N. Ojo, O. Oderinlo, Corrosion inhibitive potentials of some 2H–1-benzopyran-2-one derivatives- DFT calculations Biointerface, Res. Appl. Chem., 2021, 11(6), 13968–13981.
F. Iorhuna, A.M. Ayuba, S.A. Minjibir, T.A. Nyijime, A. Ishaq, Adsorptive properties of 4-Hexyl-tertrahydro-thiopyran-1,1- diode on Al(110) and Fe(111) surface using DFT method, Alger. J. Eng. Technol., 2023, 8(2), 309-315.
S. Hadisaputra, A. Abhi Purwoko, A. Hakim, N. Prasetyo, S. Hamdiani, Corrosion Inhibition Properties of Phenyl Phthalimide Derivatives against Carbon Steel in the Acidic Medium: DFT, MP2, and Monte Carlo Simulation Studies, ACS Omega, 2022, 7(37), 33054–33066.
D.M. Jamil, A.K. Al-Okbi, S.B. Al-Baghdadi, A.A. Al-Amiery, A. Kadhim, T.S. Gaaz, A.B. Mohamad, Experimental and theoretical studies of Schiff bases as corrosion inhibitors, Chem. Cent. J., 2018, 12, 7.
A. Kokalj, On the alleged importance of the molecular electron-donating ability and the HOMO–LUMO gap in corrosion inhibition studies, Corros. Sci., 2021, 180, 109016.
M. Rbaa, M. Galai, A.S. Abousalem, B. Lakhrissi, M.E. Touhami, I. Warad, A. Zarrouk, Synthetic, spectroscopic characterization, empirical and theoretical investigations on the corrosion inhibition characteristics of mild steel in molar hydrochloric acid by three novel 8-hydroxyquinoline derivatives, Ionics, 2020, 26(1), 503–522.
N.O. Eddy, E.E. Ebenso, Quantum chemical studies on the inhibition potentials of some Penicillin compounds for the corrosion of mild steel in 0.1 M HCl, J. Mol. Model., 2010, 16, 1291–1306.
A.A. Siaka, N.O. Eddy, S.O. Idris, L. Magaji, Z.N. Garba, I.S. Shabanda, Quantum Chemical Studies of Corrosion Inhibition and Adsorption Potentials of Amoxicillin on Mild Steel in HCl Solution, International Journal of Modern Chemistry, 2013, 4(1), 1-10
C.B. Erma, M.A. Quraishi, E.E. Ebenso, I.B. Obot, A. El Assyry, 3-amino alkylated indoles ascorrosion inhibitors for mild steel in 1M HCl: Experimental and theoretical studies, Jour-nal of Molecular Liquids, 2016, 219, 647-660
A. Tazouti, N. Errahmany, M. Rbaa, M. Galai, Z. Rouifi, R. Touir, A. Zarrouk, S. Kaya, M.E. Touhami, B. El Ibrahimi, S. Erkan, Effect of hydrocarbon chain length for acid corrosion inhibition of mild steel by three 8-(n-bromo-Ralkoxy) quinoline derivatives: experimental and theoretical investigations, J. Mol. Struct., 2021, 1244, 1–19.
A.M. Ayuba, N.A. Thomas, N.U. Shehu, F. Iorhuna, Exploring the Inhibition Potential of Carbamodithionic acid on Fe (111) Surface: A Theoretical Study, J. Eng. Ind. Res., 2024, 4(4), 201-210.
M.E. Belghiti, S. Echihi, A. Dafali, Y. Karzazi, M. Bakasse, H. Elalaoui-Elabdallaoui, L.O. Olasunkanmi, E.E. Ebenso, M. Tabyaoui, Computational simulation and statistical analysis on the relationship between corrosion inhibition efficiency and molecular structure of some hydrazine derivatives in phosphoric acid on mild steel surface, Applied Surface Science, 2019, 491, 707-22.
D. Llache Robledo, B. Berner Perez, A.G. Gonzales Guiterrez, R. Flores-Moreno, N. Casillas, DFT as a tool for predicting corrosion inhibition capacity, ECS Trans., 2021, 101(1), 277–290.
A. Singh, K.R. Ansari, P. Banerjee, M. Murmu, M.A. Quraishi, Y. Lin, Corrosion inhibition behavior of piperidinium based ionic liquids on Q235 steel in hydrochloric acid solution: experimental, density functional theory and molecular dynamics study, Colloids Surfaces A Physicochemical Eng. Asp., 2021, 623, 1–12.
A.A. Muhammed, F. Iorhuna, T.A. Nyijime, H. Muhammadjamiu, M. Sani, Exploring 4- Aminnaphthalene Derivatives for Corrosion Inhibition through Density Functional Theory and Simulation on Iron Surface, J. Eng. Ind. Res., 2024, 5(1), 1-15.
DOI: http://dx.doi.org/10.13171/mjc02407171793iorhuna
Refbacks
- There are currently no refbacks.
Copyright (c) 2024 Mediterranean Journal of Chemistry
