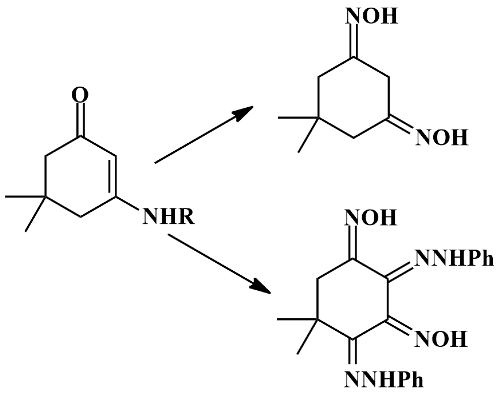
A novel trans-amination process in 3-arylamino- 5,5-dimethylcyclohex-2-en-1-one with nucleophiles and antimicrobial activity of selected products
Abstract
Full Text:
PDFReferences
- L. Yang, Y. Wu, Y. Yang, C. Wen, J-P. Wan, Catalyst-free synthesis of 4-acyl-NH-1,2,3-triazoles by water-mediated cycloaddition reactions of enaminones and tosyl azide, Beilstein J. Org. Chem., 2018, 14, 2348-2353. J.V. Greenhill, I. Chaaban, P. J. Steel, Functionalized Enaminones and their use in Heterocyclic Synthesis, J. Heterocycl. Chem., 1992, 29, 1375-1383.
- Y. Guo, Y. Xiang, J-P. Wang, Thermoinduced free-radical C-H acyloxylation of tertiary enaminones: catalyst-free synthesis of acyloxyl chromones and enaminones, Org. Lett., 2018, 20, 3971-3974. D.V. Tinh, M. Fischer, W. Stadlbauer, Ring Closure Reactions of Cyclic 2-Arylaminomethylene-1,3-diones, J. Heterocycl. Chem.1996, 33, 905- 910.
- A-Z.A. Elassar, A.A. El-Khair, Recent Developments in the Chemistry of Enaminones, Tetrahedron 2003, 59, 8463-8480.
- S.A. Shawali, T.A. Farghaly, A.R. Al dahshoury, Synthesis, Reactions and Antitumor Activity of New β-Aminovinyl-3-Pyrazolyl Ketones, Arkivoc, 2009, (xiv), 88-99.
- S.M. Al-Mousawi, M.A. El-Apasery, M.H. Elnagdi, Enaminones in Heterocyclic Synthesis: A Novel Route to Tetrahydro-pyrimidines, Dihydropyridines, Triacylbenzenes and NaphthofuransUnder Microwave Irradiation, Molecules, 2010, 15, 58-67.
- B. Govindh, B.S. Diwakar, Y.L.N. Murthy, A Brief Review on Synthesis &Applications of β-Enamino Carbonyl Compounds, Org. Commun., 2012, 105-119.
- A.A. E-H. Hassan, Heterocyclic Synthesis via Enaminones: Synthesis and Molecular Docking Studies of Some Novel Heterocyclic Compounds Containing Sulfonamide Moiety, Inter. J. Org. Chem., 2014, 4, 68-81.
- F. Al-Omran, A. El-Khair, The Behavior of 3-Anilinoenone and N-Phenyl Cinnamamide towards Carbon Nucleophiles: Spectroscopy and X-Ray Studies Reveal Interesting New Synthesis Routes to Nicotinonitriles and Tetrahydropyridine-3- Carbonitrile, Org. Chem. Curr. Res., 2014, 3, 123.
- Y.N. Mabkhot, F.D. Aldawsri, S.S. Al-Showman, A. Barakat, S.M. Soliman, M. I. Choudhary, S. Yousuf, M.S. Mubarak, T.B. Hadda, Novel Enaminone Derived from Thieno[2,3-b]Thiene: Synthesis, X-Ray Crystal Structure, HOMO, LUMO, NBO Analyses and Biological Activity, Chem. Central J. 2015, 9.
- I.H. El-Azab, L.M.Break, Z.A.A.El-Zahrani, Syntheses of Enaminone-Based Heterocyclic Compounds and Study their Biological Activity, Orien. J. Chem., 2016, 32, 2435-2449.
- N.D. Eddington, D.S. Cox, R.R. Robert, J.P. Stables, C.B. Powell, K.R. Scott, Enaminones- Versatile Therapeutic Pharmacophores. Further Advances, Curr. Med. Chem., 2000, 7, 417-436.
- I.O. Edafiogho, O.A. Phillips, E.E. Udo, S. Santosh, B. Rethish, Synthesis, Antibacterial and Anticonvulsant Evaluations of Some Cyclic Enaminones, Eur. J. Med. Chem. 2009, 44, 967-975.
- E.S.H. El Ashry, L.F. Awad, E.I. Ibrahim, O. Kh. Bdeewy, Synthesis of Antipyrine Derivatives Derived from Dimedone, Chinese J. Chem., 2007, 25, 570-573.
- E.S.H. El Ashry, L.F. Awad, E.I. Ibrahim, O. Kh. Bdeewy, Microwave Irradiation for Accelerating the Synthesis of Acridine and Xanthene Derivatives from Dimedone, Arkivoc, 2006, (ii), 178-186.
- N. Rashed, M. Sayed, E.S.H. El Ashry, Dibenzo[b,e][1,4]diazepin-1-one, Synthesis and Derivatization, J. Chinese Chem. Soc., 1993, 40, 189-194.
- H. Abdel Hamid, A. Moussad, M. Sayed, E.S.H. El Ashry, Synthesis of 2-Aryl-4,5,6,7-Tetrahydro-6,6-Dimethyl-2H-Benzo[d][1,2,3]Triazol-4-ones, Org. Prep. Proceed. Int. 1993, 25, 569-575.
- E.S.H. El Ashry, G.H. Labib, M. Shaban, F. El Sayed. Reactions of Hydrazines with Dimedones, Indian J. Chem. 1978, 16, 871-875.
- E.S.H. El Ashry, L.F. Awad, Y. El Kilany, E.I. Ibrahim, Chapter 1 Dimedone: A Versatile Precursor for Annulated Heterocycles, Adv. Heterocycl. Chem., 2009, 98, 1-141.
- J.V. Greenhill, Aromatic Enaminones. Part 1. Ultraviolet Absorption of N-Aryl Enaminones Derived from Dimedone, J. Chem. Soc. Perkin Trans 1, 1976, 2207-2210.
- M. P. Sushmita, A. K. Verma, Copper-catalyzed stereo- and chemoselective synthesis of enaminones via Michael type addition, J. Chem. Sci., 2018, 130:70 R. Dalpozzo, A. De Nino, M. Nardi, B. Russo, A. Procopio, Erbium(III) Triflate: A Valuable Catalyst for the Synthesis of Aldimines, Ketimines, and Enaminones, Synthesis, 2006, 1127-1132.
- R.S. Bhosale, P.A. Suryawanshi, S.A. Ingle, M. N. Lokhande, S.P. More S.B. Man, S.V. Bhosale, Ionic Liquid Promoted Synthesis of
β-Enamino Ketones at Room Temperature, Synlett, 2006, 6, 933-935.
- B. Datta, M.B.M. Reddy, M.A. Pasha, Molecular Iodide- Catalyzed Mild and Effective Synthesis of β-Enaminones at Room Temperature, Synth. Commun., 2011, 41, 2331-2336.
- M.A. Harrad, B. Boualy, L. El Rirdoussi, M.A.Ali, Aluminum Phosphate Catalyzed Free Solvent Preparation of β- Enamino Esters, Am. J. Chem., 2012, 2, 271-276.
- N.D. Koduri, H. Scoot, B. Hileman, J.D. Cox, M. Coffin, L. Glicksberg, S.R. Hussaini, Ruthenium Catalyzed Synthesis of Enaminons, Org. Lett., 2012, 14, 440-443.
- M. Zhang, A. Abdukader, Y. Fu, C. Zhu, Efficient Synthesis of β-Enaminones and β-Enaminoesters Catalyzed by Gold(I)/ Silver (I) under Solvent-Free Conditions, Molecules, 2012, 17, 2812-2822.
- M. Nisar, I. Ali, M.R. Shah, M. Qayum, M. Ui-Haq, U. Rashid, M. Islam, Efficient PPA-SiO2, Catalyzed Synthesis of β-Enaminones under Solvent-Free Conditions, Molecules, 2013, 18, 15182-15192.
- 27. R. Khajuria, Y. Saini, K.K. Kapoor, Ferric Nitrate Nonahydrate as a Mild and Efficient Catalyst for the synthesis of β- Enaminones, Indian J. Chem. 2014, 53B, 1122-1127.
- C-L. Feng, N-N. Chu, S-G. Zhang, J. Cai, J-Q. Chen, H-Y.Hu, M. Ji, Solvent- Free Synthesis of β-Enamino Ketones and Esters Catalyzed by RecyclableIron (III) Triflate, Chem. Papers, 2014, 68, 1097-1103.
- B. Cui, R.U Wang, L.Z. Chen, Y Jin, G.F. Han, Synthesis of Enaminones in Aqueous Media Using Catalytic Dilute HCl, Synth. Commun., 2011, 41, 1064-1070.
- S.U. Tekale, V.B. Jadhav, S.B. Munde, R.P. Pawar, Grinding Induced Solvent Free, Catalyst Free Synthesis of β-Enaminones and β- Enamino Esters Der Chem. Sinica, 2015, 6, 38-41.
- A. Kotali, V.P. Papageorgiou, Oxidation of the Dioximes of 1,3-Diketones with Lead Tetra- Acetate, J. Chem. Soc. Perk. Trans,1, 1985, 2083-2086.
- S.I. Zav,yalov, V.M. Medvedeva, Chemistry of 1,3-Cyclohexanedione, Russ. Chem. Bull., 1959, 8, 2062-2066.
- N.N. Makhova, L. L. Fershtat, Recent advances in the synthesis and functionalization of 1,2,5-oxadiazole 2-oxides, Tetrahedron Lett., 2018, 59, 2317-2326. M. Boiani, H. Cerecetto, M. Gonzalez, M. Risso, C. Olea-Azae, O.H. Piro, E.E. Castellano, A.L. de Cerain, O. Ezpeleta, A. Monge-Vega, A. 1,2,5-Oxadiazole N-Oxide Derivatives as Potential Anti-Cancer Agents: Synthesis and Biological Evaluation. Part IV, Eur. J. Med. Chem., 2001, 36, 771-782.
- A. Vaidya, S. Jain, P. Jain, P. Jain, N. Tiwari, R. Jain, R. Jain, A. K. Jain, R. K. Agrawal, Synthesis and biological activities of oxadiazole derivatives: a review, Mini. Rev. Med. Chem., 2016, 16, 835-845. G. Aguirre, L. Boiani, H. Cerecetto, R. Di Maio, M. Gonzalez, W. Porcal, A. Denicola, M. Moller, L. Thomson, V. Tortora, Benzo[1,2-c]1,2,5-Oxadiazole N-Oxide Derivatives as Potential Antitrypanosomal Drugs. Part 3: Substituents- Clustering Methodology in the Search for New Active Compounds, Bioorg. Med. Chem., 2005, 13, 6324-6335.
- L. Keurulainen, M. Heiskari, S.Nenonen, A. Nasereddin, D. Kopelyanskiy, T. O. Leino, J. Y-Kauhaluoma, C. L. Jaffe, P. Kiuru, Synthesis of carboxyimidamide-substituted benzo[c][1,2,5]oxadiazoles and their analogs and evaluation of biological activity against Leishmania donovani, Med. Chem. Comm., 2015, 6, 1673-1678.G.C. Tron, F. Pagliai, E. Del Grosso, A.A. Genazzani, G. Sorba, Synthesis and Cytotoxic Evaluation of Combrefurazans, J. Med. Chem., 2005, 48, 3260-3268.
- K. Gajula, T. Maringanti, D. Biradar, V. Chamle, R. Alvala, R. Manchal, Synthesis and antibacterial activity of novel 4-phenyl-3-( (quinoxalin-2-yloxy)methyl)-1,2,5-oxadiazole 2-oxide derivatives, J. Chem. Pharm. Res., 2018, 10, 9-14 C. Velazquez, P.N.P. Rao, R. McDonald, E.E. Knaus, Synthesis and Biological Evaluation of 3,4-Diphenyl-1,2,5-Oxadiazole-2-Oxides and 3,4-Diphenyl-1,2,5- Oxadiazoles as Potential Hybrid COX-2 Inhibitor/Nitric Oxide Donor Agents, Bioorg. Med. Chem., 2005, 13, 2749-2757.
- R.A. Olofson, J.S. Michelman, Furazan, J. Org. Chem., 1965, 30, 1854-1859.
- Y. Kamitori, A Convenient and Facile Synthesis of 3-Trifluoromethyl-1,2,5-Oxadiazoles with the Use of Silica Gel as an Effective Catalyst, Heterocycles, 1999, 51, 627-630.
- K.W.J. Baker, A.R. March, S. Parsons, R.M. Paton, 3,5-Dipyranosyl-1,2,5-Oxadiazole-2-Oxides: Synthesis and X-Ray Structure, Tetrahedron, 2002, 58, 8505-8513.
- L.S. Constantinova, S.A. Amelichev, S.G. Zlotin, M.I. Struchkova, T.I. Godovikova, O.A. Rakitin, [1,4]Dithiino[2,3-c:5,6-c,]Bis[1,2,5]Oxadiazole Di-N-Oxide: Synthesis and Oxidation to Mono- and Bis-S- Oxides, Mendeleev Commun., 2015, 25, 339-340.
- M.G.B. Drew, B. Vickery, G.R. Willy, Phenylhydrazone Derivatives of Dimedone: Hydrogen Bonding, Spectral (13C and 1H Nuclear Magnetic Resonance) and Conformational Consideration. Crystal and Molecular Structures of 5,5-Dimethylcyclohexane-1,2,3-Trione 2-(4-methylphenylhydrazone) (1) and 5,5-Dimethylcyclohexane-1,2,3-Trione 2-(4-Nitro-phenylhydrazone) (2), J. Chem. Soc. Perkin Trans., 1982, 2, 1297-1303
- P. Simunek, A. Lycka, V. Machacek, Synthesis, 1H, 13C, and 15N NMR Study of Azo Coupling from Enaminones, Eur. J. Org. Chem., 2002, 2764-2769
- P. Simunek, L. Luskova, M. Svboodova, V. Bertolasi, A. Lycka, V. Machacek, Synthesis and Structure of Some Azo Coupled Cyclic β-Enaminones Magn. Reson. Chem., 2007, 45,330-339.
- S.R. Jain, A. Kar, The Antibacterial Activity of Some Essential Oils and their Combinations, Planta Med., 1971, 20, 118-123.
- I. Jirovsky, Studies on Enaminoketones, Can. J. Chem., 1974, 52, 55-65.
DOI: http://dx.doi.org/10.13171/mjc7619019140eshea
Refbacks
- There are currently no refbacks.
Copyright (c) 2019 Mediterranean Journal of Chemistry
